Determining the Pairing for Lewis Structures
Moderators: Chem_Mod, Chem_Admin
-
ALegala_2I
- Posts: 102
- Joined: Thu Jul 11, 2019 12:17 am
Determining the Pairing for Lewis Structures
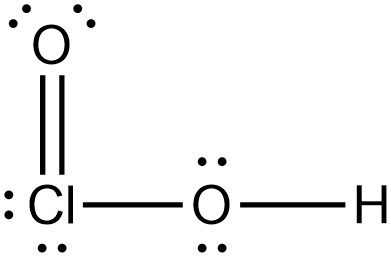
How do we know when the hydrogen will be attached to the central atom vs other atoms? Like in the case of HClO2, why is the hydrogen attached to the oxygen and not the chlorine?


-
Suraj Doshi 2G
- Posts: 100
- Joined: Fri Aug 02, 2019 12:15 am
Re: Determining the Pairing for Lewis Structures
I believe that specifically for this case, the hydrogen bonds to oxygen due to the strong covalent bond attraction. Since Cl is larger than O, the dipole-dipole attraction is weaker and thus hydrogen does not like to bond with chlorine.
-
Mashkinadze_1D
- Posts: 87
- Joined: Sat Aug 24, 2019 12:15 am
Re: Determining the Pairing for Lewis Structures
If I am correct, hydrogen generally tries to bond to N, O, or F as this allows it to take place in hydrogen bonding. Therefore, it did not bond to Cl because then the overall strength of the molecule would be decreased significantly.
Return to “Resonance Structures”
Who is online
Users browsing this forum: No registered users and 5 guests

