Difference between molecular shape
Moderators: Chem_Mod, Chem_Admin
-
Amy Luu 2G
- Posts: 105
- Joined: Wed Sep 18, 2019 12:19 am
Difference between molecular shape
What is the difference between trigonal pyramidal and T-shaped? Similarly, what is the difference between seesaw and tetrahedral? I keep choosing one but the answer is actually the other.
-
Mashkinadze_1D
- Posts: 87
- Joined: Sat Aug 24, 2019 12:15 am
Re: Difference between molecular shape
A trigonal planar shape has no lone pairs attached to the central atom and a T shaped has two lone pairs attached to the central atom. Similarly, a tetrahedral structure has no lone pairs attached to the central atom, while a seesaw would have one lone pair attached to the central atom. Hope this helps!
-
Sanjana K - 2F
- Posts: 102
- Joined: Sat Sep 07, 2019 12:17 am
- Been upvoted: 1 time
Re: Difference between molecular shape
Seesaws: 1 LP, seesaw molecular shape, trigonal bipyramidal electron arrangement, bond angles of 90 and 120 degrees
Tetrahedral: 0 LP, tetrahedral molecular shape, tetrahedral electron arrangement, bond angles of 109.5 degrees
Trigonal pyramidal: 1 LP, trigonal pyramidal molecular shape, tetrahedral electron arrangement, bond angles of 109.5 degrees
T-shape: 2 LP, T-shape molecular shape, trigonal bipyramidal electron arrangement, bond angles of 90 and 120 degrees
Tetrahedral: 0 LP, tetrahedral molecular shape, tetrahedral electron arrangement, bond angles of 109.5 degrees
Trigonal pyramidal: 1 LP, trigonal pyramidal molecular shape, tetrahedral electron arrangement, bond angles of 109.5 degrees
T-shape: 2 LP, T-shape molecular shape, trigonal bipyramidal electron arrangement, bond angles of 90 and 120 degrees
-
Sean Cheah 1E
- Posts: 105
- Joined: Wed Sep 18, 2019 12:20 am
Re: Difference between molecular shape
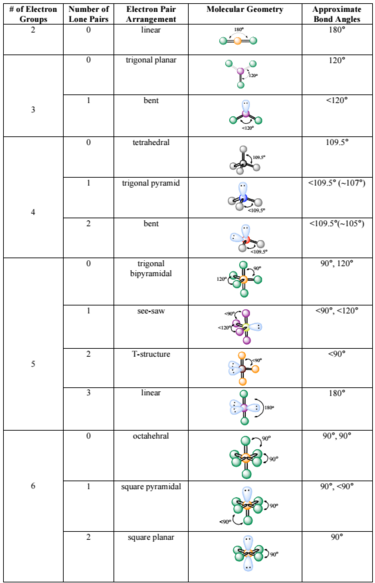
I found this simple table online that might help you visualize and remember all the various molecular geometries.

Return to “Determining Molecular Shape (VSEPR)”
Who is online
Users browsing this forum: No registered users and 8 guests

