axial vs equitorial
Moderators: Chem_Mod, Chem_Admin
-
HanaAwad_4B
- Posts: 45
- Joined: Fri Aug 30, 2019 12:18 am
axial vs equitorial
I'm kind of confused on the difference between axial and equatorial and what we need to know about them. Thank you!
-
MBouwman_4A
- Posts: 40
- Joined: Wed Sep 18, 2019 12:21 am
Re: axial vs equitorial
Axial bonds are parallel to the axis of the ring while equatorial bonds are perpendicular to the axis (at the equator).
-
Julie Park 1G
- Posts: 100
- Joined: Thu Jul 25, 2019 12:15 am
Re: axial vs equitorial
It's important to know that an axial or equatorial lone pair (e-) placement can determine the most stable structure. Ideally, you would want a structure with lower "energy" (less e- repulsion)
For example, in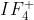 , there are two possible places (orientations) where the lone pair can exist (axial or equatorial)
, there are two possible places (orientations) where the lone pair can exist (axial or equatorial)
The axial lone pair lies will the axis of the molecule, where it strongly repels the e- pairs in the three equatorial bonds.
An equatorial lone pair will sit on the molecule’s equator (plane perpendicular to axis) where it strongly repels only the e- pairs in the two axial bonds.
In this case, because the lone pair only repels two e- pairs in the equatorial position (meaning that it has lower energy compared to the axial position) this orientation is more stable and preferred.
For example, in
The axial lone pair lies will the axis of the molecule, where it strongly repels the e- pairs in the three equatorial bonds.
An equatorial lone pair will sit on the molecule’s equator (plane perpendicular to axis) where it strongly repels only the e- pairs in the two axial bonds.
In this case, because the lone pair only repels two e- pairs in the equatorial position (meaning that it has lower energy compared to the axial position) this orientation is more stable and preferred.
-
Jillian C 4C
- Posts: 51
- Joined: Sat Jul 20, 2019 12:16 am
Re: axial vs equitorial
An axial lone pair is at the axis of the molecule while an equatorial lone pair lies at the equator. The lone pair at the equator is more favorable as it has lower energy and is more stable.
Return to “Determining Molecular Shape (VSEPR)”
Who is online
Users browsing this forum: No registered users and 13 guests

