can someone explain when we are allowed to cancel out the x to make solving the quadratic equation easier?
I'm referring to when we set up the equation after making the ice box.
Cancelling out the X
Moderators: Chem_Mod, Chem_Admin
-
Jasmine Kim 1L
- Posts: 71
- Joined: Fri Aug 02, 2019 12:16 am
Re: Cancelling out the X
The whole point of the quadratic equation is to solve for x.
After making the equation equal to 0, if it looks like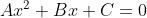 , then you can plug the coefficients into the formula
, then you can plug the coefficients into the formula 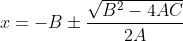 and find the value of x.
and find the value of x.
After making the equation equal to 0, if it looks like
-
Osvaldo SanchezF -1H
- Posts: 122
- Joined: Wed Sep 18, 2019 12:21 am
Re: Cancelling out the X
I want to assume that you refer to the problem when instead of having a quadratic we instead have a cubic equation. We have no way of solving for such an instance when the formula is a cubic one. So we will focus on what the change of the initial reactant will be. We will notice that the change is so small (by looking at the K value given) that it can be considered negligible. So we would just consider the initial concentration of the recant to be that at equilibrium and solve for concentration of the product.
Hope this the helps.
Hope this the helps.
-
Jessica Li 4F
- Posts: 115
- Joined: Fri Aug 09, 2019 12:16 am
Re: Cancelling out the X
You are allowed to cancel out the x, or deem it negligible to get an approximation of a K value or concentration of a reactant/product, if the K value is very small. Usually the cutoff is 10^-4, so if it is less than that, you can solve the equation without including the x. This usually happens when you have equations that might involve x^3.
Return to “Non-Equilibrium Conditions & The Reaction Quotient”
Who is online
Users browsing this forum: No registered users and 8 guests

