Autoprotolysis
Moderators: Chem_Mod, Chem_Admin
-
Clara Cho 2K
- Posts: 103
- Joined: Wed Sep 18, 2019 12:18 am
Re: Autoprotolysis
In autoprotolysis a proton is transferred between two identical molecules, one of which releases a proton which is accepted by the other molecule. Ex. H20 + H20 -> h30+ + OH-
-
aishwarya_atmakuri
- Posts: 101
- Joined: Sat Jul 20, 2019 12:15 am
-
Diana Andrade_4F
- Posts: 50
- Joined: Tue Nov 05, 2019 12:18 am
-
Michellekim1H
- Posts: 25
- Joined: Wed Feb 20, 2019 12:17 am
Re: Autoprotolysis
It basically means that a proton is transferred between the same molecule. For example, 2H2O(l) <-> H30+(aq) + OH-(aq).
-
Emily Lo 1J
- Posts: 50
- Joined: Thu Sep 19, 2019 12:16 am
Re: Autoprotolysis
It is the transfer of a proton between the same type of molecule. An example of it would be 2H2O 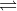 H3O
H3O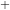 + OH
+ OH
-
Nick Lewis 4F
- Posts: 111
- Joined: Wed Sep 18, 2019 12:18 am
Re: Autoprotolysis
Lavelle talked about autoprotolysis a lot throughout lectures, so I wouldnt be surprised to see it show up on the test. Definitions above are good, i would say definitely know the equation and be able to explain it, which shouldnt be that hard if you have a rough understanding of what it is.
-
Isabella Dal Porto 1H
- Posts: 100
- Joined: Fri Aug 30, 2019 12:16 am
Re: Autoprotolysis
It is helpful to know since the autoprotolysis constant of water, Kw, is used to calculate hydronium and hydroxide concentration. That helps us determine if the solution is more acidic or basic.
-
Maya Pakulski 1D
- Posts: 105
- Joined: Thu Jul 11, 2019 12:17 am
Re: Autoprotolysis
Emily Lo 1J wrote:It is the transfer of a proton between the same type of molecule. An example of it would be 2H2OH3O
+ OH
What are some other common examples of autoprotolysis besides water?
-
Madelyn Romberg 1H
- Posts: 102
- Joined: Tue Oct 02, 2018 12:16 am
Re: Autoprotolysis
Autoprotolysis is proton transfer between the same type of molecule. In this class, water was the example. 2H20 <-> h3o+ + oh-
-
Zoe Gleason 4F
- Posts: 51
- Joined: Tue Jul 23, 2019 12:15 am
Re: Autoprotolysis
Maya Pakulski 1D wrote:Emily Lo 1J wrote:It is the transfer of a proton between the same type of molecule. An example of it would be 2H2OH3O
+ OH
What are some other common examples of autoprotolysis besides water?
Ammonia and acetic acid can also both undergo autoprolysis.
-
Madeline Phan 1E
- Posts: 103
- Joined: Sat Aug 17, 2019 12:18 am
Re: Autoprotolysis
Autoprotolysis occurs when a proton is transferred between two identical molecules.
Re: Autoprotolysis
Protolysis is a process that occurs with polyprotic substances. There are occurrences where a molecule can give another of the same type of molecule a proton.
-
Lizette Noriega 1H
- Posts: 105
- Joined: Wed Sep 18, 2019 12:15 am
-
William Chan 1D
- Posts: 102
- Joined: Sat Sep 14, 2019 12:15 am
Re: Autoprotolysis
It is proton transfer between two of the same molecule. For example, if we have two water molecules, one can donate a proton to the other, and result in OH- and H30+ molecules.
Return to “Phase Changes & Related Calculations”
Who is online
Users browsing this forum: No registered users and 4 guests

