internal energy
Moderators: Chem_Mod, Chem_Admin
-
Savannah Mance 4G
- Posts: 107
- Joined: Fri Aug 30, 2019 12:17 am
internal energy
When a question asks what is the change in the internal energy of a reaction, what are they asking you to find? Are they asking for the work?
-
Morgan Carrington 2H
- Posts: 54
- Joined: Wed Nov 14, 2018 12:22 am
Re: internal energy
Internal energy is represented as 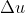 so depending on the question and what values are given, you would use the equation (or its variants)
so depending on the question and what values are given, you would use the equation (or its variants) 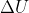 = q + w.
= q + w.
-
Veronica_Lubera_2A
- Posts: 106
- Joined: Sat Sep 14, 2019 12:16 am
- Been upvoted: 1 time
Re: internal energy
Also another derivation of the formula we learned in class today was DeltaU=DeltaH-P(DeltaV) if the pressure is constant.
-
DanielTalebzadehShoushtari2A
- Posts: 53
- Joined: Fri Aug 02, 2019 12:16 am
Re: internal energy
Veronica_Lubera_2A wrote:Also another derivation of the formula we learned in class today was DeltaU=DeltaH-P(DeltaV) if the pressure is constant.
And if the volume is constant, work is equal to 0 because the DeltaV in the work term is 0. So, in that case, DeltaU = q
When a question asks you to find change in internal energy, they are asking you to find DeltaU, and depending on the conditions given in the questions, the calculation of it will be different. In general, it is given by DeltaU = q +w
-
Caroline Beecher 2H
- Posts: 51
- Joined: Wed Nov 14, 2018 12:21 am
Re: internal energy
Internal energy, U, is a state property and functions from its final and initial states. For example, if a closed system is changed by heating (+q) and compression (+w) then the equation for internal energy is: delta U = q + w. If volume is constant, delta U = q(v).
Who is online
Users browsing this forum: No registered users and 9 guests

