Phase Changes
Moderators: Chem_Mod, Chem_Admin
-
Leslie Almaraz 4G
- Posts: 99
- Joined: Fri Aug 02, 2019 12:16 am
-
Ashley Tran 2I
- Posts: 108
- Joined: Thu Jul 11, 2019 12:17 am
Re: Phase Changes
Yes, during a phase change, the temperature doesn't change because the energy is channeled into breaking the intermolecular forces of the substance.
-
Junwei Sun 4I
- Posts: 125
- Joined: Wed Oct 02, 2019 12:16 am
Re: Phase Changes
That’s correct because during a phase change energy is used to overcome the intermolecular forced between molecules instead of raising the actual temperature of the substance.
-
Nick Fiorentino 1E
- Posts: 102
- Joined: Wed Sep 18, 2019 12:16 am
Re: Phase Changes
The temperature must be high enough to cause the phase change but not high enough to cause the system to change temperature
-
Maya Pakulski 1D
- Posts: 105
- Joined: Thu Jul 11, 2019 12:17 am
-
Orrin Zhong 4G
- Posts: 51
- Joined: Sat Jul 20, 2019 12:16 am
Re: Phase Changes
Phase changes occur at constant temperature because the energy put into the system is breaking the intermolecular forces between the molecules rather than increasing the kinetic energy of the molecules.
-
Bella Townsend
- Posts: 50
- Joined: Wed Feb 20, 2019 12:18 am
Re: Phase Changes
If you look at a heating curve, the slanted lines represent the temperature increasing, and the flat lines represent the phase change where there is no change in temperature. For example, on the first straight line, the leftmost point represents solid water at 0 degrees celsius, and on the most right point of the first flat line represents 0 degrees celsius for liquid water.
-
Sanjana Borle 2K
- Posts: 111
- Joined: Fri Aug 09, 2019 12:15 am
Re: Phase Changes
Phase Changes occur when temperature is constant, and the temperature goes up as the entropy of the solution goes up; however it doesn't undergo a phase change at this time.
Re: Phase Changes
Yes it is constant because the energy is going towards breaking the forces of a molecule so that such a change can occur.
Re: Phase Changes
Looking on a heating curve, as the temperature reaches a certain point, it will remain constant before increasing again. That constant temperature is where the phase change occurs.
-
Rebekah Alfred 1J
- Posts: 102
- Joined: Thu Jul 11, 2019 12:15 am
- Been upvoted: 1 time
Re: Phase Changes
Bella Townsend wrote:If you look at a heating curve, the slanted lines represent the temperature increasing, and the flat lines represent the phase change where there is no change in temperature. For example, on the first straight line, the leftmost point represents solid water at 0 degrees celsius, and on the most right point of the first flat line represents 0 degrees celsius for liquid water.
Here's a visual that goes along with Bella's explanation:
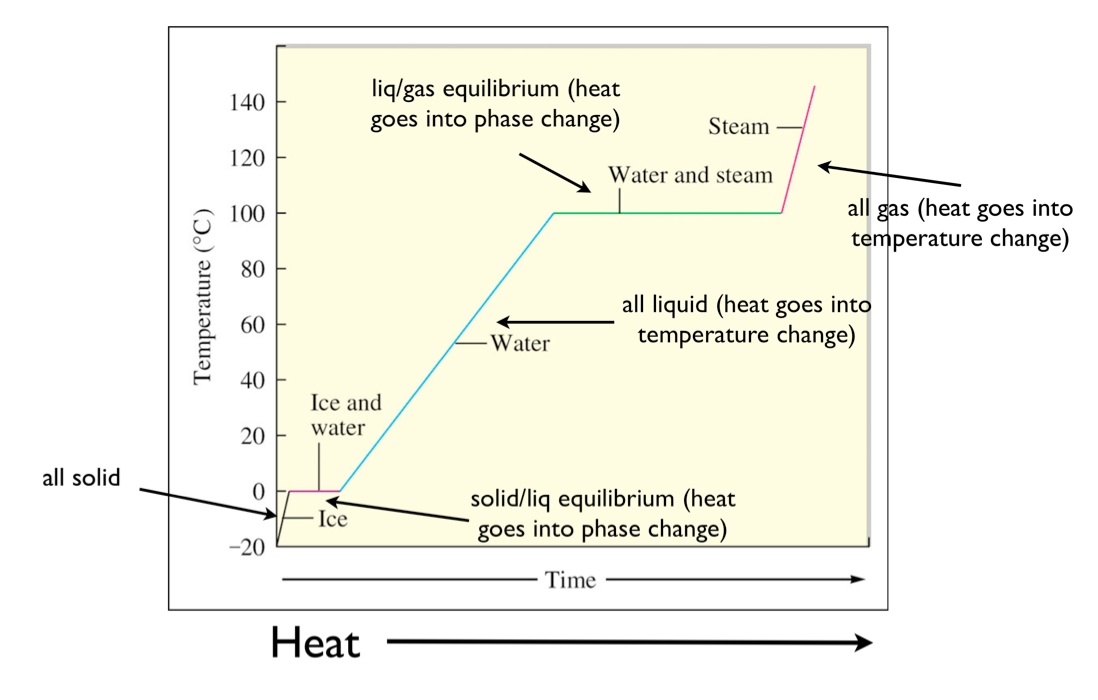
-
Simon Dionson 4I
- Posts: 107
- Joined: Sat Sep 14, 2019 12:17 am
Re: Phase Changes
Phase changes are constant to change intermolecular forces. Sloped lines can indicate heat capacities.
-
Catherine Daye 1L
- Posts: 104
- Joined: Wed Sep 11, 2019 12:16 am
Re: Phase Changes
Yes, because the energy from the temperature is used to break/create bonds between molecules for their phase change.
-
Nuoya Jiang
- Posts: 99
- Joined: Sat Sep 14, 2019 12:17 am
Re: Phase Changes
Yes, during phase change, the temperature remains constant, but latent heat is released or absorbed.
-
Jessica Katzman 4F
- Posts: 35
- Joined: Mon Jun 17, 2019 7:24 am
Re: Phase Changes
The temperature does not change during a phase change- for example, you can have both ice and water at the same temperature.
-
Madelyn Romberg 1H
- Posts: 102
- Joined: Tue Oct 02, 2018 12:16 am
Re: Phase Changes
Temperature does not change during a phase change. However, there is still energy being put into the system. The energy is going into breaking intermolecular bonds rather than thermal heat.
-
Jesse Anderson-Ramirez 3I
- Posts: 54
- Joined: Thu Sep 26, 2019 12:18 am
Re: Phase Changes
While a phase change is occurring the temperature is constant, but once the phase change is complete the temperature will continue to change.
-
lauraxie2e
- Posts: 108
- Joined: Fri Aug 09, 2019 12:17 am
Return to “Phase Changes & Related Calculations”
Who is online
Users browsing this forum: No registered users and 10 guests

