Isolated vs Closed [ENDORSED]
Moderators: Chem_Mod, Chem_Admin
-
Rita Chen 1B
- Posts: 112
- Joined: Sat Jul 20, 2019 12:15 am
Isolated vs Closed
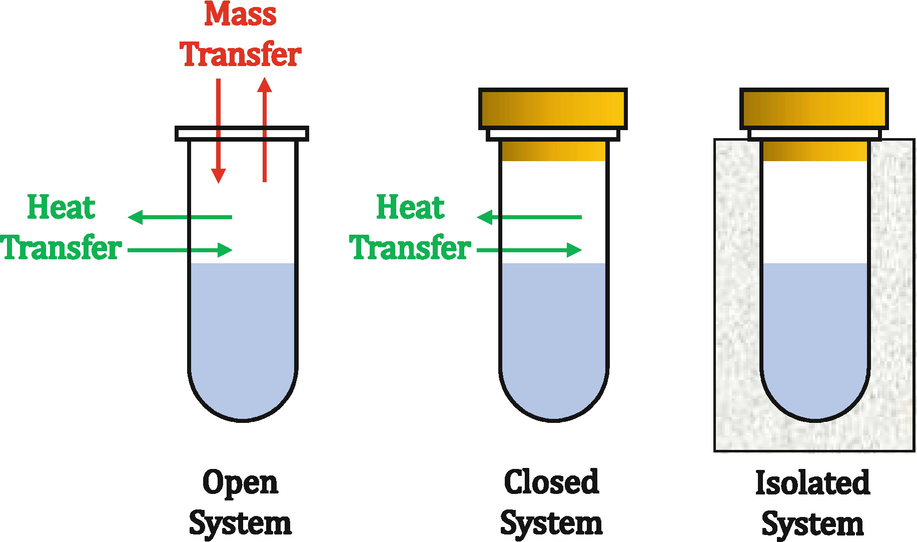
How can we tell which one is which? Like does an insulated water bottle count as isolated?
-
Jasmine Fendi 1D
- Posts: 108
- Joined: Sat Aug 24, 2019 12:15 am
Re: Isolated vs Closed
A closed system is a sealed system but energy can be exchanged with the surrounding. On the other hand, an isolated system is completely isolated from the environment and it cannot be affected by the surroundings. That being said, an insulated bottle is an isolated system if we assume that it is completely insulated and the outside environment cannot change the system.
-
LBacker_2E
- Posts: 104
- Joined: Wed Sep 18, 2019 12:21 am
- Been upvoted: 1 time
Re: Isolated vs Closed
When something is insulate that means there is not a relationship with the surroundings, and therefore it would be an isolated system. An example of an isolated system is something that occurs in a bomb calorimeter.
Re: Isolated vs Closed
If something is specified as being insulated and rigid, that means it doesn't exchange heat or work of expansion with the surroundings. This makes the system isolated. If these are not specified, but the system doesn't allow the exchange of matter with the surroundings, then the system of closed.
-
Amanda Mei 1B
- Posts: 109
- Joined: Sat Aug 24, 2019 12:16 am
Re: Isolated vs Closed
A closed system is physically closed but not thermodynamically closed, meaning that although matter cannot be exchanged between the system and its surroundings, energy can. An example would be a sealed beaker of water, which doesn't insulate, or a pan with a lid on top of it on the stove. An isolated system is both physically and thermodynamically closed. One example is a thermos, which insulates and doesn't allow transfer of energy (heat) or the physical contents inside.
-
Kylie Lim 4G
- Posts: 110
- Joined: Sat Aug 17, 2019 12:15 am
Re: Isolated vs Closed
In an open system, both heat and matter can be exchanged with the system's surroundings. In a closed system, energy but not matter can be exchanged, and in an isolated system neither can be exchanged with the surroundings. If the insulated water bottle has a lid and prevents heat from escaping or entering the system, it would be considered isolated.
-
Ying Yan 1F
- Posts: 101
- Joined: Fri Aug 02, 2019 12:16 am
Re: Isolated vs Closed
Yes, an insulated water bottle would definately be a isolated system because no heat nor matter can be transfered. On the other hand, a normal water bottle would be an example of a closed system because while matter cannot be transfered, heat still can be transfered.
-
Hope Hyland 2D
- Posts: 50
- Joined: Wed Feb 20, 2019 12:16 am
Re: Isolated vs Closed
In a closed system, heat can still be transferred, but in an isolated system, neither heat nor matter can be transferred.
Re: Isolated vs Closed
An open system can exchange energy and matter with its surroundings. A closed system can exchange energy but not matter with its surroundings. An isolated system cannot exchange energy and matter with its surroundings. Therefore, an insulated system is an isolated system.
-
Edmund Zhi 2B
- Posts: 118
- Joined: Sat Jul 20, 2019 12:16 am
Re: Isolated vs Closed
An insulated water bottle can be considered isolated because neither matter nor energy can be exchanged with the surroundings
-
Nuoya Jiang
- Posts: 99
- Joined: Sat Sep 14, 2019 12:17 am
Re: Isolated vs Closed
Yes, it is isolated in an ideal situation because we can neither exchange substance nor energy with the surrounding.
-
Jessica Katzman 4F
- Posts: 35
- Joined: Mon Jun 17, 2019 7:24 am
Re: Isolated vs Closed
A closed system is sealed, but can still interact with the outside environment. An isolated system is sealed and insulated and cannot interact with the environment.
Re: Isolated vs Closed
By strict definition, there is no such thing as an isolated system. So, things that come close to being isolated count as isolated—a thermos would.
-
Kishan Shah 2G
- Posts: 132
- Joined: Thu Jul 11, 2019 12:15 am
Re: Isolated vs Closed
An isolated system is something where matter and energy cannot enter or leave the system. A closed system is where only energy, but not matter, can enter or leave the system. And an open system is where both matter and energy can enter or leave the system. That being said, an insulated water bottle means that no matter can leave the water bottle, and since it is insulated no energy can either, so its isolated.
-
RobertXu_2J
- Posts: 104
- Joined: Fri Aug 30, 2019 12:17 am
Re: Isolated vs Closed
If it says it is insulated, we would assume that it is isolated. I don't think the wording of questions will be ambiguous. If it looks like the question is trying to imply that the system does not exchange energy (e.g. heat) with the environment, then it's probably trying to tell you that it is an isolated system. On the other hand, if it does not mention anything about the lack of energy transfer, then I think it would be safe to assume that it is closed.
-
kausalya_1k
- Posts: 50
- Joined: Wed Nov 14, 2018 12:23 am
Re: Isolated vs Closed
An open system is where energy and matter can be exchanged. A closed system is where energy can be exchanged, and an isolated system is where nothing can be exchanged between the system and surroundings.
-
TimVintsDis4L
- Posts: 104
- Joined: Sat Aug 17, 2019 12:17 am
Re: Isolated vs Closed
Open System ( matter & energy can exchange with surroundings)
Beaker of Water Water can evaporate & Beaker does not insulate
Closed System( energy can exchange with surroundings)
Sealed beaker of water Beaker does not insulate
Isolated System ( nothing exchanged with surroundings)
Combustion of glucose in a bomb calorimeter
This is what I got from lecture, hope this helps.
Beaker of Water Water can evaporate & Beaker does not insulate
Closed System( energy can exchange with surroundings)
Sealed beaker of water Beaker does not insulate
Isolated System ( nothing exchanged with surroundings)
Combustion of glucose in a bomb calorimeter
This is what I got from lecture, hope this helps.
-
William Francis 2E
- Posts: 101
- Joined: Wed Sep 18, 2019 12:20 am
Re: Isolated vs Closed
The biggest difference to me seems to be that isolated systems include insulation. For instance, a completely sealed water bottle is a closed system (since it can still exchange energy with the surroundings through the medium of the bottle’s material), but a well-insulated bottle is an isolated system (since it can’t exchange energy with the surroundings).
Re: Isolated vs Closed
Open systems allow everything (matter and heat) to go in and out. Closed systems only allow heat to go in and out. Isolated systems don't allow either in or out.
-
Kylie Lim 4G
- Posts: 110
- Joined: Sat Aug 17, 2019 12:15 am
Re: Isolated vs Closed
In a closed system, matter cannot be transferred but heat energy can. In an isolated system the transfer of both heat energy and matter are contained in the system.
Re: Isolated vs Closed
In an isolated system, the system cannot exchange matter or energy with the surroundings. In a closed system, the system has a fixed amount of matter but it can exchange energy with the surroundings. if the water bottle is insulated really well then it will be an isolated system because it will not be able to exchange matter or energy with its surroundings.
-
Leslie Almaraz 4G
- Posts: 99
- Joined: Fri Aug 02, 2019 12:16 am
Re: Isolated vs Closed
in a closed system, the matter is constant yet energy can be exchanged with the surroundings whereas in the isolated system neither matter nor energy is exchanged.
-
Rebekah Alfred 1J
- Posts: 102
- Joined: Thu Jul 11, 2019 12:15 am
- Been upvoted: 1 time
-
Kate Swertfager
- Posts: 50
- Joined: Wed Sep 18, 2019 12:17 am
Re: Isolated vs Closed
If the system doesn’t exchange energy(heat) or matter then it is isolated. If it exchanges Energy(heat) but not matter then it is closed. If it exchanges both then it is open.
An isolated water bottle doesn’t exchange heat with surroundings or matter so it is isolated.
An isolated water bottle doesn’t exchange heat with surroundings or matter so it is isolated.
-
Diana Andrade_4F
- Posts: 50
- Joined: Tue Nov 05, 2019 12:18 am
Re: Isolated vs Closed
A closed system is when a system can only exchange energy (like heat) with its surroundings. Meanwhile, an isolated system is a system that can't exchange anything with its surroundings.
-
Jocelyn Thorp 1A
- Posts: 103
- Joined: Wed Sep 18, 2019 12:20 am
Re: Isolated vs Closed
though you can never truly have an isolated system, if a problem says that the system is insulated (especially if it mentions being well insulated) then it's probably best to consider it isolated
-
Astrid Lunde 1I
- Posts: 103
- Joined: Sat Sep 07, 2019 12:16 am
Re: Isolated vs Closed
Closed system can exchange heat while an isolated system has no effect from the surroundings.
-
Emily Lo 1J
- Posts: 50
- Joined: Thu Sep 19, 2019 12:16 am
Re: Isolated vs Closed
For an isolated system, nothing can come in or out of the system. In a closed system, heat can come in and out of the system. Both can't have matter come in or out but the main difference between isolated and closed is that nothing can come inside an isolated system. It's like an insulated bottle, heat stays in but the heat from the liquid doesn't leave the system.
-
Nick Lewis 4F
- Posts: 111
- Joined: Wed Sep 18, 2019 12:18 am
Re: Isolated vs Closed
WIll it always be specified if the item is insulated? Like are we expected to know certain things they reference are insulated? What if its hard to distinguish, or is it pretty clear whether or not its insulated?
-
Rosa Munoz 2E
- Posts: 105
- Joined: Wed Sep 18, 2019 12:21 am
-
Caroline Zepecki
- Posts: 101
- Joined: Fri Aug 09, 2019 12:16 am
Re: Isolated vs Closed
If a water bottle is vacuum sealed, then technically heat is not escaping so no energy is being exchanged with the surrounding environment
-
saigorijavolu2k
- Posts: 108
- Joined: Sat Sep 28, 2019 12:15 am
Re: Isolated vs Closed
A closed system can only exchange energy with its surroundings.
An isolated system does not exchange anything.
Open can exchange heat and matter.
Think of a bomb calorimeter as an isolated system and a closed system would be like an open pan on stove, where energy is getting exchanged but not matter
An isolated system does not exchange anything.
Open can exchange heat and matter.
Think of a bomb calorimeter as an isolated system and a closed system would be like an open pan on stove, where energy is getting exchanged but not matter
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: Isolated vs Closed [ENDORSED]
saigorijavolu2k wrote:A closed system can only exchange energy with its surroundings.
An isolated system does not exchange anything.
Open can exchange heat and matter.
Think of a bomb calorimeter as an isolated system and a closed system would be like an open pan on stove, where energy is getting exchanged but not matter
You mean a closed system would be like an closed/sealed pan on stove, where energy is exchanged but not matter.
-
Juana Abana 1G
- Posts: 100
- Joined: Wed Sep 18, 2019 12:15 am
Re: Isolated vs Closed
An insulated water bottle can be considered an isolated system because it can neither exchange any substances nor energy with its surrounding.
Return to “Thermodynamic Systems (Open, Closed, Isolated)”
Who is online
Users browsing this forum: No registered users and 5 guests