How to interpret reversible/irreversible graphs
Moderators: Chem_Mod, Chem_Admin
-
Andrew F 2L
- Posts: 103
- Joined: Sat Aug 17, 2019 12:17 am
How to interpret reversible/irreversible graphs
For Gibbs free energy, what I understand is that when the value of Delta G is below 0, the reaction is reversible. How do I interpret the graph of the reversible reaction from there? Why is temperature constant along the pathway according to Dr Lavelle? Thank you
-
Kate Osborne 1H
- Posts: 102
- Joined: Fri Aug 30, 2019 12:16 am
Re: How to interpret reversible/irreversible graphs
I think the temperature is constant because most reactions occur at a constant external temperature and pressure
-
Madelyn Romberg 1H
- Posts: 102
- Joined: Tue Oct 02, 2018 12:16 am
Re: How to interpret reversible/irreversible graphs
For a reversible reaction, it occurs under constant temperature because it is such a slow and small change that it remains balanced between the internal and external environment.
-
Jessica Katzman 4F
- Posts: 35
- Joined: Mon Jun 17, 2019 7:24 am
Re: How to interpret reversible/irreversible graphs
The temperature is constant for reversible reactions
Re: How to interpret reversible/irreversible graphs
The temperature is considered "constant" for reversible actions, but not because it doesn't change at all. It's constant because the change is so slow that at an instantaneous moment, it is not really changing. Plus, the system temp and the surrounding temp stay the same as each other.
-
Shivam Rana 1D
- Posts: 106
- Joined: Fri Aug 09, 2019 12:16 am
Re: How to interpret reversible/irreversible graphs
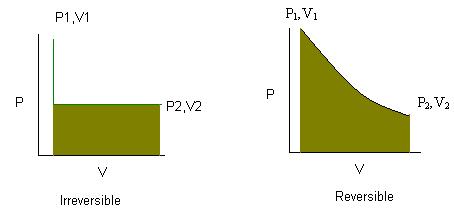
For a pressure versus volume graph a reversible process, which is usually isothermic, will be a curved line. An irreversible reaction will be a straight line.
-
Eva Zhao 4I
- Posts: 101
- Joined: Sun Sep 29, 2019 12:16 am
Re: How to interpret reversible/irreversible graphs
Something to note is that the work a system can do is greatest in a reversible process, hence the larger area under the curve for the reversible graph. The graphs are attached to this for your convenience.
Return to “Gibbs Free Energy Concepts and Calculations”
Who is online
Users browsing this forum: No registered users and 14 guests

