C in nCv ln (T2/T1)
Moderators: Chem_Mod, Chem_Admin
-
Jason Wu 1E
- Posts: 101
- Joined: Thu Jul 25, 2019 12:15 am
C in nCv ln (T2/T1)
What is the C represent? I thought it was a heat capacity but why is it capitalized? is it a constant? or does it depend on the compound at hand? Also when is it applicable for us to use the equation?
-
KaleenaJezycki_1I
- Posts: 127
- Joined: Sat Aug 17, 2019 12:18 am
- Been upvoted: 2 times
Re: C in nCv ln (T2/T1)
C in Cv is heat capacity and will most likely be the heat capacity of a monoatomic gas : Cv= 3/2R
-
Leslie Almaraz 4G
- Posts: 99
- Joined: Fri Aug 02, 2019 12:16 am
-
Leslie Almaraz 4G
- Posts: 99
- Joined: Fri Aug 02, 2019 12:16 am
Re: C in nCv ln (T2/T1)
there is also a different way to find Cv and Cp for mono and diatomic molecules.
-
Sanjana Munagala_1j
- Posts: 103
- Joined: Sat Aug 24, 2019 12:17 am
- Been upvoted: 1 time
Re: C in nCv ln (T2/T1)
You need to also be cautious when you have a system that has an entropy change due to both a temperature change and a volume change. When you calculate the entropy change for just the temperature change you have to make the assumption that the volume is constant.
Hope that helps!
Hope that helps!
-
Eva Zhao 4I
- Posts: 101
- Joined: Sun Sep 29, 2019 12:16 am
Re: C in nCv ln (T2/T1)
To add on, the relationship between Cv and Cp is Cp = Cv + 1. The values for Cv are shown below (know that we look at the ideal gas values):
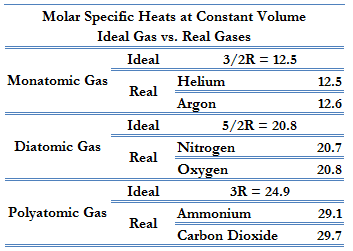

-
Adriana_4F
- Posts: 74
- Joined: Fri Sep 28, 2018 12:29 am
Re: C in nCv ln (T2/T1)
Eva Zhao 4I wrote:To add on, the relationship between Cv and Cp is Cp = Cv + 1. The values for Cv are shown below (know that we look at the ideal gas values):
It's not one it is actually R, the gas constant. Cp=Cv + R
-
Adriana_4F
- Posts: 74
- Joined: Fri Sep 28, 2018 12:29 am
Return to “Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)”
Who is online
Users browsing this forum: No registered users and 4 guests

