test problem 4A.5 continued
Moderators: Chem_Mod, Chem_Admin
-
JosephineF
- Posts: 49
- Joined: Wed Sep 18, 2019 12:17 am
test problem 4A.5 continued
Why does this problem use 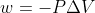 for an irreversible expansion but w=-nRTln(V2/V1) for a reversible expansion?
for an irreversible expansion but w=-nRTln(V2/V1) for a reversible expansion?
-
LBacker_2E
- Posts: 104
- Joined: Wed Sep 18, 2019 12:21 am
- Been upvoted: 1 time
Re: test problem 4A.5 continued
You always use w=Pex x (delta)V for an irreversible expansion because the change in volume is measurable. For a reversible process you use the other equation because the change in volume is infinitestimally small. For the reversible expansion, pressure falls as the volume increases which is reflected by the nRT part of the equation from PV=nRT.
-
Grace Jansen 2A
- Posts: 28
- Joined: Sat Jul 20, 2019 12:16 am
Re: test problem 4A.5 continued
If you look back at the notes, you can see Dr. Lavelle derive the equation for the reversible expansion. Additionally, it is also in the book.
-
Ivan Tadeja 1G
- Posts: 81
- Joined: Fri Sep 28, 2018 12:28 am
Re: test problem 4A.5 continued
I'm not exactly sure of the derivations for both, but I would just correlate each equation to irreversible and reversible respectively. Just know which of those equations to use given its reversible or not.
Return to “Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)”
Who is online
Users browsing this forum: No registered users and 1 guest

