Final Exam #15
Moderators: Chem_Mod, Chem_Admin
-
Anuradha S 1F
- Posts: 50
- Joined: Fri Sep 27, 2019 12:29 am
Final Exam #15
On the final, #15 asked us to find  ionization for H20 --> OH- + H+. How were we supposed to find this? I googled the
ionization for H20 --> OH- + H+. How were we supposed to find this? I googled the  formation of H2O and OH- and used
formation of H2O and OH- and used 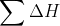 products -
products - 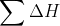 reactants. Was this the right way to go about it or did we have to use the pH or
reactants. Was this the right way to go about it or did we have to use the pH or  fus some how?
fus some how?
-
Prasanna Padmanabham 4I
- Posts: 111
- Joined: Thu Jul 25, 2019 12:17 am
Re: Final Exam #15
I tend to forget stressful scenarios, so I don't remember what was on the final lol, but I believe I used the Van't Hoff equation.
The equation relates delta H, two equilibrium and two different temperatures together. I used information given in the previous equation for the K values and T values. Using those values I solved for H...
Page 432 in the textbook goes over the Van't Hoff equation and used different "forms" from the page to answer the questions on that section of the test...
but I don't know if that was the right way....I'm sure Dr. Lavelle will publish answer soon and I'll know for sure if my method was right.
The equation relates delta H, two equilibrium and two different temperatures together. I used information given in the previous equation for the K values and T values. Using those values I solved for H...
Page 432 in the textbook goes over the Van't Hoff equation and used different "forms" from the page to answer the questions on that section of the test...
but I don't know if that was the right way....I'm sure Dr. Lavelle will publish answer soon and I'll know for sure if my method was right.
-
Eugene Chung 3F
- Posts: 142
- Joined: Wed Nov 15, 2017 3:03 am
Re: Final Exam #15
Anuradha S 1F wrote:On the final, #15 asked us to findionization for H20 --> OH- + H+. How were we supposed to find this? I googled the
formation of H2O and OH- and used
products -
reactants. Was this the right way to go about it or did we have to use the pH or
fus some how?
I believe I used the van't hoff equation..
lnK = -deltaH/RT + deltaS/R or ln(K2/K1) = deltaH(rxn)/ R (1/T1 - 1/T2)
-
Ayushi2011
- Posts: 101
- Joined: Wed Feb 27, 2019 12:17 am
Who is online
Users browsing this forum: No registered users and 6 guests

