Light hits a sodium metal surface and the velocity of the ejected electron is 6.61 x 10^5 m.s^-1. The work function for sodium is 150.6 kJ.mol^-1. Answer the following three questions.
A. What is the kinetic energy of the ejected electron?
B. How much energy is required to remove an electron from one sodium atom?
C. What is the frequency of the incident light on the sodium metal surface?
I watched the video module and I'm still confused. I assume for part A we use E= hV - work function. I'm not sure what to do for part B and for part C I think we use E(photon) = threshold energy + Ek? Can someone break this problem down and explain for me?
(I think I should've posted this in properties of electrons but I have no clue how to edit that)
Photoelectron Effect: POST/PRE Module Assessment #28-30
Moderators: Chem_Mod, Chem_Admin
-
JaylinWangDis1L
- Posts: 102
- Joined: Wed Sep 30, 2020 10:09 pm
-
John Pham 3L
- Posts: 149
- Joined: Wed Sep 30, 2020 9:49 pm
- Been upvoted: 4 times
Re: Photoelectron Effect: POST/PRE Module Assessment #28-30
For A, you should use the KE equation.
- KE =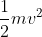 .
.
- Just plug in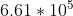 m/s and mass of Electron
m/s and mass of Electron 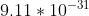 kg to solve
kg to solve
For B, you are given the amount of energy needed in KJ per mole.
- Convert from KJ to Joules
- Convert moles to atoms using Avogadro's number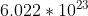 atoms
atoms
- You should end up with Joules per Sodium atom
For C, you need to first calculate the KE from part A and the energy required for one Sodium atom from part B.
- Use E(Photon) = E(Threshold Energy) + E(Kinetic Energy) to solve for Photon energy
- Take Photon's energy and plug into E = hv, solving for frequency
- KE =
- Just plug in
For B, you are given the amount of energy needed in KJ per mole.
- Convert from KJ to Joules
- Convert moles to atoms using Avogadro's number
- You should end up with Joules per Sodium atom
For C, you need to first calculate the KE from part A and the energy required for one Sodium atom from part B.
- Use E(Photon) = E(Threshold Energy) + E(Kinetic Energy) to solve for Photon energy
- Take Photon's energy and plug into E = hv, solving for frequency
-
Yun Su Choi 3G
- Posts: 109
- Joined: Wed Sep 30, 2020 10:09 pm
Re: Photoelectron Effect: POST/PRE Module Assessment #28-30
For Part C, I followed through those steps, but I still got the wrong answer. Can someone point out where I went wrong?
Ephoton= 1.506 x 10^5 + 1.99x10^-19 (answer from part a)
Ephoton= 150600
E=hv
V=E/h
V= 150600/ (6.626 x 10^-34)
V= 2.27 x 10^38
Ephoton= 1.506 x 10^5 + 1.99x10^-19 (answer from part a)
Ephoton= 150600
E=hv
V=E/h
V= 150600/ (6.626 x 10^-34)
V= 2.27 x 10^38
-
John Pham 3L
- Posts: 149
- Joined: Wed Sep 30, 2020 9:49 pm
- Been upvoted: 4 times
Re: Photoelectron Effect: POST/PRE Module Assessment #28-30
Yun Su Choi 2I wrote:For Part C, I followed through those steps, but I still got the wrong answer. Can someone point out where I went wrong?
Ephoton= 1.506 x 10^5 + 1.99x10^-19 (answer from part a)
Ephoton= 150600
You haven't converted from Joule per mole to Joule per atom
1.506*10^5 is still in Joules per mole. Just divide by Avogadro's constant to get Joule per atom.
Then you can plug in v = E / h to solve for frequency
-
Madison Muggeo 3H
- Posts: 106
- Joined: Wed Sep 30, 2020 9:35 pm
Re: Photoelectron Effect: POST/PRE Module Assessment #28-30
Thank you for this thread! I got stuck on #29 but this really helped!
-
Tiao Tan 3C
- Posts: 109
- Joined: Wed Sep 30, 2020 9:59 pm
Re: Photoelectron Effect: POST/PRE Module Assessment #28-30
John Pham 2B wrote:Yun Su Choi 2I wrote:For Part C, I followed through those steps, but I still got the wrong answer. Can someone point out where I went wrong?
Ephoton= 1.506 x 10^5 + 1.99x10^-19 (answer from part a)
Ephoton= 150600
You haven't converted from Joule per mole to Joule per atom
1.506*10^5 is still in Joules per mole. Just divide by Avogadro's constant to get Joule per atom.
Then you can plug in v = E / h to solve for frequency
Hi. I am a little confused with C too. From which part of the question did you tell that we need to convert to Joule per atom? or is it because question B asked for the energy to remove AN ELECTRON, and question c is a follow-up?
-
Andrew Jubintoro 3J
- Posts: 123
- Joined: Wed Sep 30, 2020 9:58 pm
- Been upvoted: 1 time
Re: Photoelectron Effect: POST/PRE Module Assessment #28-30
When you use E=hv to solve for the frequency remember that the Energy here is the energy of a single photon. And one photon can only eject one electron (from one atom). Therefore, E(photon) = E(to remove one e-) + E(excess energy in the form of kinetic energy)
Return to “Properties of Light”
Who is online
Users browsing this forum: No registered users and 4 guests

