I still don't completely understand that idea, why were we able to change the negative sign to a positive one? Also why do we need to change it?
Lecture 7 Question [ENDORSED]
Moderators: Chem_Mod, Chem_Admin
-
clairehathaway 2J
- Posts: 112
- Joined: Wed Sep 30, 2020 9:38 pm
Lecture 7 Question
In today's lecture when working through the example asking for the frequency of light emitted by a hydrogen atom going from n=4 to n=2, we use the equation 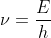 and when we input the energy value (E) we change it from negative to positive (the negative value was equal to delta E). Dr. Lavelle says that the sign change because the change in energy for the electron and for the photon it's equal and opposite.
and when we input the energy value (E) we change it from negative to positive (the negative value was equal to delta E). Dr. Lavelle says that the sign change because the change in energy for the electron and for the photon it's equal and opposite.
I still don't completely understand that idea, why were we able to change the negative sign to a positive one? Also why do we need to change it?
I still don't completely understand that idea, why were we able to change the negative sign to a positive one? Also why do we need to change it?
-
Lucy Wang 2J
- Posts: 101
- Joined: Wed Sep 30, 2020 10:09 pm
- Been upvoted: 2 times
Re: Lecture 7 Question
The negative sign simply is to show that this is the amount of energy LOST by the electron. We changed it to a positive sign to show the amount of energy being ABSORBED by the photon or turned into light. This works because according to the conservation of energy, energy cannot be created or destroyed. So basically, while the electron is losing this energy, it is passing it on to the photon which allows the energy lost from the electron to be shown as light.
To give an analogy, its like making a payment to someone. In your account (electron) it may show -$2.00, but in their account (photon) it would be +$2.00. lol not sure if that makes sense but I hope it was helpful
To give an analogy, its like making a payment to someone. In your account (electron) it may show -$2.00, but in their account (photon) it would be +$2.00. lol not sure if that makes sense but I hope it was helpful
-
Nayra Gharpetian 3F
- Posts: 101
- Joined: Wed Sep 30, 2020 9:40 pm
- Been upvoted: 1 time
Re: Lecture 7 Question [ENDORSED]
yes the analogy is a perfect example. the negative sign is to show that the electron is losing energy, but we change it to a positive to calculate for wavelength and frequency, etc. basically to prove the conservation of energy.
-
Sana Nagori 2H
- Posts: 149
- Joined: Wed Sep 30, 2020 9:43 pm
- Been upvoted: 1 time
Re: Lecture 7 Question
Lucy Wang 1A wrote:To give an analogy, its like making a payment to someone. In your account (electron) it may show -$2.00, but in their account (photon) it would be +$2.00. lol not sure if that makes sense but I hope it was helpful
This analogy is very helpful, Thanks Lucy!
-
clairehathaway 2J
- Posts: 112
- Joined: Wed Sep 30, 2020 9:38 pm
Re: Lecture 7 Question
Lucy Wang 1A wrote:The negative sign simply is to show that this is the amount of energy LOST by the electron. We changed it to a positive sign to show the amount of energy being ABSORBED by the photon or turned into light. This works because according to the conservation of energy, energy cannot be created or destroyed. So basically, while the electron is losing this energy, it is passing it on to the photon which allows the energy lost from the electron to be shown as light.
To give an analogy, its like making a payment to someone. In your account (electron) it may show -$2.00, but in their account (photon) it would be +$2.00. lol not sure if that makes sense but I hope it was helpful
That makes so much sense, thank you so much!
Return to “Bohr Frequency Condition, H-Atom , Atomic Spectroscopy”
Who is online
Users browsing this forum: No registered users and 1 guest

