Nodal planes
Moderators: Chem_Mod, Chem_Admin
-
jordanginyard_
- Posts: 101
- Joined: Wed Sep 30, 2020 9:43 pm
Nodal planes
What are nodal planes and how do they relate to quantum numbers. Are they any videos I can watch that relates to what the teachers want us to understand from this topic.
-
Ryan_Page_1J
- Posts: 100
- Joined: Wed Sep 30, 2020 10:03 pm
- Been upvoted: 1 time
Re: Nodal planes
A nodal plane is where the probability of finding an electron is 0 (i.e. no electrons can exist in the nodal plane). The s orbital has no nodal planes due to its spherical nature.
-
manisha_joseph_1H
- Posts: 101
- Joined: Wed Sep 30, 2020 10:01 pm
- Been upvoted: 1 time
Re: Nodal planes
A nodal place is where the probability of finding an electron is 0. For instance, s orbitals have no nodal planes, which therefore leads to a symmetric electron density distribution. However, any orbital beyond this (such as p, d, or f) all have nodal planes, which leads to a non-symmetric electron distribution.
-
Christine Ma 3L
- Posts: 102
- Joined: Wed Sep 30, 2020 9:35 pm
Re: Nodal planes
To add on, the number of nodal planes, for the most part, should be equal to the angular momentum quantum number 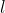 .
.
So when l=0, there are no nodal planes, l=1 has 1 nodal plane, etc. Hope this helps!
So when l=0, there are no nodal planes, l=1 has 1 nodal plane, etc. Hope this helps!
-
Keerthana Sundar 1K
- Posts: 103
- Joined: Wed Sep 30, 2020 9:52 pm
Re: Nodal planes
In terms of the diagrams of the various orbitals, is the nodal plane simply the area which is not bounded by the orbital drawings? Or is there something more? Will we be expected to point out where nodal planes are for various orbitals? Thanks!
Return to “Quantum Numbers and The H-Atom”
Who is online
Users browsing this forum: No registered users and 5 guests

