Sapling #7
Moderators: Chem_Mod, Chem_Admin
-
Ashley Lopez 3J
- Posts: 100
- Joined: Wed Sep 30, 2020 10:06 pm
Sapling #7
One of the answers to the question of which one was polar was CH2CL2. When it is drawn, it looks like it would be non-polar and that there would no zero electric dipole. Can someone please explain why is it polar and why the dipoles do not cancel?
-
Katherine_Douglas_1F
- Posts: 88
- Joined: Wed Sep 30, 2020 10:01 pm
Re: Sapling #7
There is not a guarantee that the atoms will line up across from each other. The way it is written out, it does look as though the polar Cl-C binds and H-Cl bonds line up and are across from each other. However, this isn't necessarily the way they will line up. An H and Cl atom might line up across from each other on the C molecule. In this case, the bonds would not cancel out and so the molecule is polar.
-
Christine Ma 3L
- Posts: 102
- Joined: Wed Sep 30, 2020 9:35 pm
Re: Sapling #7
CH2Cl2 would in fact be nonpolar if the 2 C-H bonds and 2 C-Cl bonds were directly across from each other, and just by looking at the Lewis structure they do look like they would cancel out since they're drawn that way. But when you look at the molecule in 3D, CH2Cl2 is tetrahedral (4 e- regions) and, as it turns out, none of the bonds are directly across from each other so none of them actually cancel, resulting in a polar molecule.
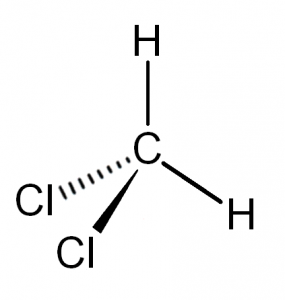

-
Hannah Alltucker 3L
- Posts: 117
- Joined: Wed Sep 30, 2020 9:44 pm
Re: Sapling #7
I believe it has something to do with the difference in polarity of the bonded atoms (the C-H and the C-Cl bonds), so they don't actually line up evenly like we think. If you actually look at a picture of what the molecule looks like when you can see it's angles, you are able to see that the two sets of bonded atoms bend towards different sides, with the H bonds bent to one and the Cl bonds bent to another. This is because there is a larger difference in electronegativity between C and Cl and there is almost no difference between C and H. Because of these differences in polarities they don't cancel out, and the molecule is therefore polar.
Return to “Determining Molecular Shape (VSEPR)”
Who is online
Users browsing this forum: No registered users and 4 guests

