ICE Box method
Moderators: Chem_Mod, Chem_Admin
-
Bronson Mathos 1H
- Posts: 104
- Joined: Wed Sep 30, 2020 9:36 pm
ICE Box method
Hello, I am having a bit of difficulty understanding how to use the ice box and was wondering if someone could explain how I would use it in these chemical equilibrium problems?
-
AdilaAhmed3I
- Posts: 101
- Joined: Wed Sep 30, 2020 10:01 pm
Re: ICE Box method
Bronson Mathos 1H wrote:Hello, I am having a bit of difficulty understanding how to use the ice box and was wondering if someone could explain how I would use it in these chemical equilibrium problems?
So an ice box is used for weak acids or bases that do not completely dissociate. There are three sections to consider: the initial concentration or pressure of the compound provided, the change in concentration/pressure, and the concentration/ pressure present at equilibrium.
So to start, let's think of a generic weak acid HA that has a molarity of .01 and write an equation for it.
HA <-> H+ + A-
Now to create an ice table:
HA <-> H+ + A-
I
C
E
We know initially we have .01 M of this weak acid. Initially, we only have the reactant and no products so the ice table should look like this:
HA <-> H+ + A-
I 0.01 0 0
C
E
Then, let's say the concentration of HA changes by some amount. For the sake of the example we are going to assume we are not given a value so we will use 'x' to denote this unknown change. We know that during the reaction, some of the reactant will be used up to form products meaning that the concentration of HA should decrease while the concentrations of H+ and A- increase:
HA <-> H+ + A-
I 0.01 0 0
C -x +x +x
E
Now at equilibrium, we will simply perform the operation denoted above:
HA <-> H+ + A-
I 0.01 0 0
C -x +x +x
E 0.01-x x x
So at equilibrium, we have the concentration of HA = 0.01-x and the concentration of H+ and A- = x.
Now we would set up our equilibrium constant expression. Since this is a weak acid, we use Ka.
Ka= ([H+][A-])/[HA]
If you are not told the change in concentration, you will usually be given a Ka value. You would substitute in that Ka value in the expression above and replace the concentrations with the ones you found in the ice table and solve for x.
Ka= ([x][x])/[0.01-x]
-
Fiona Huang 3C
- Posts: 56
- Joined: Wed Nov 25, 2020 12:19 am
Re: ICE Box method
The ice box is just a helpful way of keeping track of changing concentrations or pressures or each specifies in an equilibrium reaction. The I stands for initial concentration/pressure, the C stands for the change in concentration/pressure, and the E is what the value is when the system is at equilibrium.
You can use any units as long as the units stay consistent throughout the table, but I prefer using mol/L because it’s more organized and prevents careless mistakes at the end. Typically, you are given the initial concentrations so you put that down for the first row in the table in mol/L. You assign a variable “x” to represent the change in concentration over the course of the reaction.
Next you determine which way the reaction is proceeding. For example, if it is proceeding forward, the reactants will decrease (so the change will be -x), and the products will increase (the change will be +x). Using the K value, which is the equilibrium constant, you can set up the equation with the x variables, set it equal to K, and solve for the x to find the concentrations at equilibrium.
You can use any units as long as the units stay consistent throughout the table, but I prefer using mol/L because it’s more organized and prevents careless mistakes at the end. Typically, you are given the initial concentrations so you put that down for the first row in the table in mol/L. You assign a variable “x” to represent the change in concentration over the course of the reaction.
Next you determine which way the reaction is proceeding. For example, if it is proceeding forward, the reactants will decrease (so the change will be -x), and the products will increase (the change will be +x). Using the K value, which is the equilibrium constant, you can set up the equation with the x variables, set it equal to K, and solve for the x to find the concentrations at equilibrium.
-
Ayesha Aslam-Mir 3C
- Posts: 122
- Joined: Wed Sep 30, 2020 9:43 pm
Re: ICE Box method
Hi Bronson, I will try my best to help out here. I learned it as RICE (adding an R for the line with the reaction) but it's essentially the same concept. Remember generally using an ICE table you'll be using concentrations, so be sure you have values in M (mol/L)
On the first line, you'll want to write the (R)eaction as to line up the concentrations of individual products and reactants you'll be looking at.
On the next line, you'll write the (I)nitial concentrations of either products and reactants that you already know.
Beneath this is the (C)hange in concentration-- usually a problem will let you know if a certain concentration is used (you could subtract an amount of a reactant) or an amount of product is produced (you would be adding).
Lastly, you'll have a line with the (E)quilibrium concentrations. You'll put together the initial concentrations and add or subtract their changes. With these new terms or amounts, you can solve for the equilibrium constant K or use a known value of K to solve for an unknown change in the concentration.
There's a goof writeup and example for ICE tables linked here:https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Equilibria/Le_Chateliers_Principle/Ice_Tables
Here's an example with the acid dissociation equation: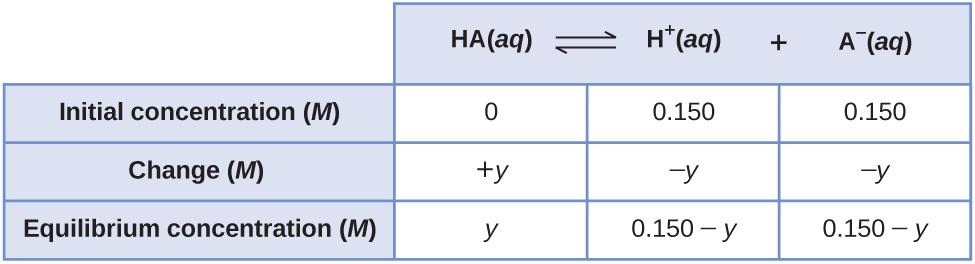
Hope that helps!
On the first line, you'll want to write the (R)eaction as to line up the concentrations of individual products and reactants you'll be looking at.
On the next line, you'll write the (I)nitial concentrations of either products and reactants that you already know.
Beneath this is the (C)hange in concentration-- usually a problem will let you know if a certain concentration is used (you could subtract an amount of a reactant) or an amount of product is produced (you would be adding).
Lastly, you'll have a line with the (E)quilibrium concentrations. You'll put together the initial concentrations and add or subtract their changes. With these new terms or amounts, you can solve for the equilibrium constant K or use a known value of K to solve for an unknown change in the concentration.
There's a goof writeup and example for ICE tables linked here:https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Equilibria/Le_Chateliers_Principle/Ice_Tables
Here's an example with the acid dissociation equation:

Hope that helps!
Last edited by Ayesha Aslam-Mir 3C on Sun Jan 17, 2021 3:40 am, edited 1 time in total.
-
Ayesha Aslam-Mir 3C
- Posts: 122
- Joined: Wed Sep 30, 2020 9:43 pm
Re: ICE Box method
AdilaAhmed3I wrote:Bronson Mathos 1H wrote:Hello, I am having a bit of difficulty understanding how to use the ice box and was wondering if someone could explain how I would use it in these chemical equilibrium problems?
So an ice box is used for weak acids or bases that do not completely dissociate. There are three sections to consider: the initial concentration or pressure of the compound provided, the change in concentration/pressure, and the concentration/ pressure present at equilibrium.
So to start, let's think of a generic weak acid HA that has a molarity of .01 and write an equation for it.
HA <-> H+ + A-
Now to create an ice table:
HA <-> H+ + A-
I
C
E
We know initially we have .01 M of this weak acid. Initially, we only have the reactant and no products so the ice table should look like this:
HA <-> H+ + A-
I 0.01 0 0
C
E
Then, let's say the concentration of HA changes by some amount. For the sake of the example we are going to assume we are not given a value so we will use 'x' to denote this unknown change. We know that during the reaction, some of the reactant will be used up to form products meaning that the concentration of HA should decrease while the concentrations of H+ and A- increase:
HA <-> H+ + A-
I 0.01 0 0
C -x +x +x
E
Now at equilibrium, we will simply perform the operation denoted above:
HA <-> H+ + A-
I 0.01 0 0
C -x +x +x
E 0.01-x x x
So at equilibrium, we have the concentration of HA = 0.01-x and the concentration of H+ and A- = x.
Now we would set up our equilibrium constant expression. Since this is a weak acid, we use Ka.
Ka= ([H+][A-])/[HA]
If you are not told the change in concentration, you will usually be given a Ka value. You would substitute in that Ka value in the expression above and replace the concentrations with the ones you found in the ice table and solve for x.
Ka= ([x][x])/[0.01-x]
the way you are awake at this fantastic time of day (night??) and still churn out these fantastic tutorials. you are a blessing
-
AdilaAhmed3I
- Posts: 101
- Joined: Wed Sep 30, 2020 10:01 pm
Re: ICE Box method
Ayesha Aslam-Mir 3C wrote:AdilaAhmed3I wrote:Bronson Mathos 1H wrote:Hello, I am having a bit of difficulty understanding how to use the ice box and was wondering if someone could explain how I would use it in these chemical equilibrium problems?
So an ice box is used for weak acids or bases that do not completely dissociate. There are three sections to consider: the initial concentration or pressure of the compound provided, the change in concentration/pressure, and the concentration/ pressure present at equilibrium.
So to start, let's think of a generic weak acid HA that has a molarity of .01 and write an equation for it.
HA <-> H+ + A-
Now to create an ice table:
HA <-> H+ + A-
I
C
E
We know initially we have .01 M of this weak acid. Initially, we only have the reactant and no products so the ice table should look like this:
HA <-> H+ + A-
I 0.01 0 0
C
E
Then, let's say the concentration of HA changes by some amount. For the sake of the example we are going to assume we are not given a value so we will use 'x' to denote this unknown change. We know that during the reaction, some of the reactant will be used up to form products meaning that the concentration of HA should decrease while the concentrations of H+ and A- increase:
HA <-> H+ + A-
I 0.01 0 0
C -x +x +x
E
Now at equilibrium, we will simply perform the operation denoted above:
HA <-> H+ + A-
I 0.01 0 0
C -x +x +x
E 0.01-x x x
So at equilibrium, we have the concentration of HA = 0.01-x and the concentration of H+ and A- = x.
Now we would set up our equilibrium constant expression. Since this is a weak acid, we use Ka.
Ka= ([H+][A-])/[HA]
If you are not told the change in concentration, you will usually be given a Ka value. You would substitute in that Ka value in the expression above and replace the concentrations with the ones you found in the ice table and solve for x.
Ka= ([x][x])/[0.01-x]
the way you are awake at this fantastic time of day (night??) and still churn out these fantastic tutorials. you are a blessing
the chem grind never stops
Return to “Equilibrium Constants & Calculating Concentrations”
Who is online
Users browsing this forum: No registered users and 6 guests

