X Approximations
Moderators: Chem_Mod, Chem_Admin
-
Daniel Hernandez 1G
- Posts: 100
- Joined: Wed Sep 30, 2020 9:45 pm
X Approximations
When given Ka or Kb, how can we determine whether or not the value is small enough to approximate the value or ‘x’ or assume that ‘x’ is near zero?
Re: X Approximations
If Ka or Kb is < 10^-4, we can be certain that the value of x is small enough. You can also check by dividing your x value by the initial concentration (of a weak acid/base) given, and if it's <5%, your approximation is valid.
-
Ashley Ko 3I
- Posts: 114
- Joined: Wed Sep 30, 2020 9:54 pm
Re: X Approximations
Hi! I believe that Professor Lavelle said that if the value of Ka/Kb is less than 10^-4, then it is safe to assume that the value of x is small enough to approximate. However, to check if your approximation is valid, divide x by the initial concentration and then multiply by 100%. If x is less than 5% of the initial concentration then the assumption is valid. Otherwise, you have to use the quadratic equation. I hope this helps!
-
Sean Wang 1F
- Posts: 100
- Joined: Wed Sep 30, 2020 9:34 pm
-
Alexandra Ahlschlager 1L
- Posts: 101
- Joined: Wed Sep 30, 2020 9:59 pm
Re: X Approximations
If the value of the equilibrium constant is less than 10^-4 you can assume that it will be small enough to approximate. You can also use the 5% rule, so if your x value is less than 5% of the initial concentration, you should be able to approximate. Hope this helps!
-
Halle Villalobos 3E
- Posts: 100
- Joined: Wed Sep 30, 2020 9:52 pm
Re: X Approximations
Hi! When values of Ka or Kb are < 10^-4, they are considered small enough that we can make the approximation of x equaling 0. I hope this helps!
-
Sarah Huh 1K
- Posts: 44
- Joined: Wed Nov 18, 2020 12:26 am
-
Kristina Krivenko 3I
- Posts: 118
- Joined: Wed Sep 30, 2020 9:52 pm
- Been upvoted: 1 time
Re: X Approximations
You can assume x value is small enough to approximate it when the equilibrium constant (whether it is Ka, Kb, etc) is smaller than or equal to 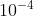 .
.
You can also double check whether using the approximation in a given case was valid by using the 5% rule. If % ionization or protonation is less than 5%, then the answer you found using the approximation method should be correct. However, it is larger than 5%, you need to go back and solve the problem using the quadratic formula to get a more correct answer.
Hope this helps :)
You can also double check whether using the approximation in a given case was valid by using the 5% rule. If % ionization or protonation is less than 5%, then the answer you found using the approximation method should be correct. However, it is larger than 5%, you need to go back and solve the problem using the quadratic formula to get a more correct answer.
Hope this helps :)
-
Kaili Valenzuela 2B
- Posts: 97
- Joined: Wed Sep 30, 2020 9:55 pm
Re: X Approximations
Hey! When Ka or Kb is less than 10^ -4 then the values are considered to be very small and we can approximate that x will equal to 0.
-
Rich Luong 1D
- Posts: 101
- Joined: Wed Sep 30, 2020 9:49 pm
Re: X Approximations
It could sometimes depend on the initial concentration whether to approximate X as zero. If the initial concentration is very small and the Ka or Kb value is very near that value, I'd say X would still play a significant role in the solution. However, I'd say if the Ka or Kb value is 10^-4 smaller than the initial concentration value, X could be rounded off to 0.
-
Stephen Min 1I
- Posts: 100
- Joined: Wed Sep 30, 2020 10:01 pm
Re: X Approximations
To add on, you can verify your assumption that x is very small by dividing the x value by the initial concentration. If the result is less than 5 percent, than the assumption is valid.
-
Sofia Lombardo 2C
- Posts: 100
- Joined: Wed Sep 30, 2020 9:31 pm
Re: X Approximations
If Ka or Kb is less than 10^-4, then we can assume x to be negligible. We can check to make sure that x is negligible by using the percent protonation/ionized by seeing if it is less than 5%. If it is greater than 5% then we cannot assume x to be negligible.
-
Alisa Nagashima 1B
- Posts: 51
- Joined: Tue Nov 17, 2020 12:19 am
Re: X Approximations
You can approximate when Ka or Kb < 10-4. You can also double check by dividing x by the initial concentration given. If less than 5%, your approximation is valid.
-
Javier Perez M 1H
- Posts: 80
- Joined: Wed Sep 30, 2020 9:52 pm
Re: X Approximations
If the Ka or Kb is < 10^-4 then it is fine to ignore the -x from the initial mols.
-
jasonfarrales3D
- Posts: 104
- Joined: Wed Sep 30, 2020 9:55 pm
Re: X Approximations
Given your Ka or Kb value, you can approximate only if their values are less than 10^-4. Furthermore, if you find the percent protonation/ionization, and it is greater than 5%, then that approximation is invalid, and you would have to go through and use the quadratic formula. If the percent protonation/ionization is less than 5%, then the approximation is valid.
-
Justin Zhang_1A
- Posts: 100
- Joined: Wed Sep 30, 2020 9:48 pm
-
Sameer Chowdhury 3C
- Posts: 102
- Joined: Wed Sep 30, 2020 9:41 pm
Re: X Approximations
So long as Ka or Kb is less than 1X10^-4 we can assume that the x is so small we can ignore the -x.
-
Levon Grigoryan
- Posts: 52
- Joined: Wed Dec 02, 2020 12:19 am
Re: X Approximations
you can always assume and instead of solving the quadratic formula ignore the x for the B and solve the equation and squareroot it to get rid of the x^2. When you get the value of the x and divide it by the initial concentration. (keep in mind the 5 percent rule). So if the value less than 5% keep going. If it is more. You go back and solve the quadratic equation to get the actually values, instead of ignoring the x for the (B)
-
Vivian_Le_1L
- Posts: 53
- Joined: Thu Dec 17, 2020 12:19 am
Re: X Approximations
You can approximate when your K value is less than 10^-4. We can assume that x is small enough that we can ignore -x. Remember that this does not mean we can ignore x^2 (I'm just adding this bit b/c someone asked about this on another post). The approximation can be checked by finding the % ionization/protonation. If it is less than 5%, then the approximation is acceptable.
-
Nan_Guan_1L
- Posts: 106
- Joined: Wed Sep 30, 2020 9:59 pm
Re: X Approximations
if you don't want to do the 5% test, you can check if Ka or Kb given is less than 10-4. usually we can use approximations if they are less than 10-4.
-
Jared Limqueco 3E
- Posts: 97
- Joined: Wed Sep 30, 2020 9:37 pm
Re: X Approximations
Ka and Kb has to be negligible, so generally < 10^-14 to omit x. Hopefully your approximation is valid if the percent ionization is <5%, otherwise you gotta do more work
-
Agustina Santa Cruz 2F
- Posts: 103
- Joined: Wed Sep 30, 2020 10:01 pm
Re: X Approximations
Usually if K is smaller than 10^-4, you can approximate and take it out of denominator so that you don't have to use the quadratic formula.
-
Sahaj Patel Lec3DisK
- Posts: 130
- Joined: Wed Sep 30, 2020 10:03 pm
- Been upvoted: 1 time
Re: X Approximations
If Ka/Kb is less than 10^-4, it is deemed as small enough, however, afterwards you calculate the percent ionization, and if it is more than 5%, the approximation is invalid and you have to do the calculation again without the approximation. Hope this helps!
-
Britney Tran IJ
- Posts: 103
- Joined: Fri Aug 30, 2019 12:18 am
Return to “Equilibrium Constants & Calculating Concentrations”
Who is online
Users browsing this forum: No registered users and 8 guests

