In a second experiment a shorter wavelength light source is used resulting in ejected electrons with a kinetic energy of 4.200 x 10-19J.
What is the energy of this incident light? What is the wavelength of this incident light?
What is the difference between the kinetic energy and just the energy of the incident light?
Given the kinetic energy, how do you find the energy? [ENDORSED]
Moderators: Chem_Mod, Chem_Admin
-
Brandon_Phan_3J
- Posts: 21
- Joined: Wed Sep 21, 2016 2:56 pm
Re: Given the kinetic energy, how do you find the energy?
I think the energy of the incident light is referring to the threshold energy added with the kinetic energy. In the course reader, the formula is E(photon) = threshold energy + Ek; E(photon) refers to the energy of the incident light and Ek refers to the kinetic energy. Hope that helps!
-
Michelle_Nguyen_3F
- Posts: 40
- Joined: Wed Sep 21, 2016 2:59 pm
Re: Given the kinetic energy, how do you find the energy? [ENDORSED]
The difference between the kinetic energy and the energy of the incident light is that the kinetic energy refers to the [b]energy of the ejected electron[/b]. This can be found using the equation 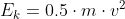 , where m = mass of the electron and v = velocity of the ejected electron. When the energy of the photon is greater than the work function of the metal (energy required to remove an electron from the metal atom), the electron is ejected with that "extra" energy. Thus, the ejected electron has kinetic energy. On the other hand, the energy of the incident light refers to the energy of the photon that caused the electron to be pushed off of the metal. The energy of the incident light, or the energy of the photon is given by
, where m = mass of the electron and v = velocity of the ejected electron. When the energy of the photon is greater than the work function of the metal (energy required to remove an electron from the metal atom), the electron is ejected with that "extra" energy. Thus, the ejected electron has kinetic energy. On the other hand, the energy of the incident light refers to the energy of the photon that caused the electron to be pushed off of the metal. The energy of the incident light, or the energy of the photon is given by 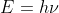 , where h = Planck's constant and
, where h = Planck's constant and 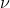 is the frequency of the incident light/photon.
is the frequency of the incident light/photon.
The main distinction is that the incident light refers to the energy of the photon that is "shone" onto the metal, and kinetic energy refers to the energy of the electron that is ejected. Hope this helps!
The main distinction is that the incident light refers to the energy of the photon that is "shone" onto the metal, and kinetic energy refers to the energy of the electron that is ejected. Hope this helps!
Re: Given the kinetic energy, how do you find the energy?
Thank you! Your explanation was very clear.
-
Camille 4I
- Posts: 57
- Joined: Sat Aug 24, 2019 12:18 am
Re: Given the kinetic energy, how do you find the energy?
In this problem, is the kinetic energy referring to the excess energy? Or is it referring to the energy needed to emit an electron?
Return to “Photoelectric Effect”
Who is online
Users browsing this forum: No registered users and 8 guests

