Sponetanity
Moderators: Chem_Mod, Chem_Admin
-
Susanna Givan 2B
- Posts: 144
- Joined: Thu Feb 27, 2020 12:16 am
Sponetanity
Can we determine spontaneity by just looking at Gibbs free energy or we also have to look at enthalpy and entropy?
-
Aaron Akhavan-Dis1B
- Posts: 106
- Joined: Wed Sep 30, 2020 9:59 pm
Re: Sponetanity
When deltaG (Gibbs) is negative, it's spontaneous. You can use the equation deltaG=deltaH - TdeltaS. and see when delta H and delta S make delta G negative. Also when deltaS is positive then the reaction is spontaneous. When deltaH is negative then the reaction is spontaneous. But again plug in the numbers into the equation to see which can be positive or negative and still get a negative deltaG.
-
LovepreetSran_3H
- Posts: 103
- Joined: Wed Sep 30, 2020 9:33 pm
Re: Sponetanity
A negative deltaG, Gibbs free energy, is spontaneous, so yes it can be used to determine spontaneity.
-
Nishan Reddy 3K
- Posts: 131
- Joined: Fri Sep 24, 2021 5:28 am
Re: Sponetanity
When ∆G is negative, the reaction is spontaneous whereas the reaction is non-spontaneous if the ∆G is positive. This relates to the enthalpy and entropy because when the ∆enthalpy is negative and the ∆entropy is positive, the reaction is ALWAYS spontaneous. Thus, we can determine spontaneity through the relationship between entropy and enthalpy which is expressed through the Gibb's free energy.
-
Brooke Gushiken 1B
- Posts: 101
- Joined: Fri Sep 24, 2021 6:48 am
Re: Sponetanity
If ΔG (Gibbs Free Energy) is negative, then the reaction will be spontaneous. Also, it's important to keep in mind that ΔG = ΔH - TΔS, so you can see which combined values for enthalpy and entropy will result in a negative ΔG and therefore spontaneous reaction.
-
Triston Dinh 1D
- Posts: 101
- Joined: Fri Sep 24, 2021 6:03 am
Re: Sponetanity
If you know the value of delta G then you will automatically know if the reaction is spontaneous. If only given the values for enthalpy and entropy, you would need to solve for delta G using the equation: delta G = delta H - T*(delta S).
Re: Sponetanity
If you know the Gibbs free energy value then you can determine spontaneity because if the free energy is negative then the reaction is spontaneous. Entropy and Enthalpy do relate to Gibbs free energy using ΔG = ΔH - TΔS so if you know those values you can also determine spontaneity as well.
-
Jessica Cornelia Hongarta 1G
- Posts: 114
- Joined: Fri Sep 24, 2021 5:49 am
Re: Sponetanity
The  itself should be enough to determine whether a reaction's spontaneity (
itself should be enough to determine whether a reaction's spontaneity (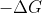 indicating that the reaction is spontaneous and
indicating that the reaction is spontaneous and 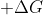 indicating that the reaction is not spontaneous. However, if you are not given
indicating that the reaction is not spontaneous. However, if you are not given  , you can use entropy and enthalpy to figure out whether the
, you can use entropy and enthalpy to figure out whether the  is positive or negative (using
is positive or negative (using 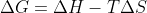 ) to determine the reaction's spontaneity.
) to determine the reaction's spontaneity.
Hope this helps!
Hope this helps!
-
Albert Chen 1G
- Posts: 101
- Joined: Fri Sep 24, 2021 6:28 am
Re: Sponetanity
When delta G is negative, the reaction is spontaneous and if positive, non-spontaneous. You can use the equation:
ΔG = ΔH - TΔS
to figure out delta g give entropy and enthalpy.
ΔG = ΔH - TΔS
to figure out delta g give entropy and enthalpy.
-
Anika Scott 3A
- Posts: 107
- Joined: Fri Sep 24, 2021 6:53 am
Re: Sponetanity
You can determine whether a reaction is spontaneous based on whether delta G is positive or negative. A negative deltaG means the reaction is spontaneous, positive = non-spontaneous. Thus, you don't need the other values to determine.
-
Emily Engelkemier 1E
- Posts: 105
- Joined: Fri Sep 24, 2021 5:10 am
Re: Sponetanity
Spontaneity is determined by delta G. If delta G is negative, then a reaction is spontaneous. If delta G is positive, then a reaction is not spontaneous.
-
Dillon Taing 3H
- Posts: 102
- Joined: Fri Sep 24, 2021 5:20 am
Re: Sponetanity
A reaction that takes place without external influence and favors product formation can be considered spontaneous. Because spontaneous reactions are exergonic, they can be identified when Gibbs free energy (ΔG) is negative, while a positive ΔG value indicates that the reaction is positive.
-
Acharya Ranawat 3E
- Posts: 102
- Joined: Fri Sep 24, 2021 6:39 am
Re: Sponetanity
Delta G is related to spontaneity. You can use delta H and delta S in order to calculate delta G. Then, if delta G is negative it is spontaneous and vice versa.
Re: Sponetanity
Delta G can be derived utilizing change in entropy/enthalpy values or by optimizing the standard value of free energy formation for products minus reactants.
-
Ivan Huang Dis 3B
- Posts: 102
- Joined: Fri Sep 24, 2021 7:32 am
-
Naomi Christian 1E
- Posts: 104
- Joined: Fri Sep 24, 2021 5:45 am
Re: Sponetanity
If delta G is negative, the reaction is spontaneous. If delta G is positive, the reaction is not spontaneous.
-
Audrey Banzali-Marks 1A
- Posts: 108
- Joined: Fri Sep 24, 2021 6:32 am
Re: Sponetanity
Since Gibbs free energy takes into account both enthalpy and entropy, we can determine spontaneity using Gibbs free energy alone. If the Gibbs free energy is negative, it's spontaneous.
-
Jordyn Lee 1J
- Posts: 106
- Joined: Fri Sep 24, 2021 7:08 am
Re: Sponetanity
You can determine spontaneity by just looking at Gibbs free energy because it incorporates enthalpy and entropy.
-
rachelsjordan 1K
- Posts: 106
- Joined: Fri Sep 24, 2021 6:35 am
-
Diana Avalos
- Posts: 55
- Joined: Tue Feb 16, 2021 12:15 am
Re: Sponetanity
Hello,
Yes looking at ΔG can determine spontaneity.
When ΔG is negative, this means the reaction is spontaneous!
Yes looking at ΔG can determine spontaneity.
When ΔG is negative, this means the reaction is spontaneous!
-
Rebekah Jung 1C
- Posts: 101
- Joined: Fri Sep 24, 2021 6:40 am
- Been upvoted: 1 time
Re: Sponetanity
If delta G is negative then the reaction is spontaenous, whereas if it is positive the reaction is non-spontaneous.
-
danielle05
- Posts: 50
- Joined: Mon Jan 03, 2022 9:19 pm
Re: Sponetanity
Gibbs free energy alone is enough to determine spontaneity. When G is negative, the reaction is spontaneous.
-
Michael 1G
- Posts: 101
- Joined: Fri Sep 24, 2021 6:16 am
- Been upvoted: 1 time
Re: Sponetanity
Gibbs free energy is what determines spontaneity. A negative delta g represents a spontaneous reaction.
-
Aparna Pillai 1E
- Posts: 109
- Joined: Fri Sep 24, 2021 7:09 am
Re: Sponetanity
Spontaneity is determined by a negative value of delta G, which is calculated by enthalpy and entropy as in the equation delta G = delta H - T * delta S.
-
Furkan Acar 3C
- Posts: 114
- Joined: Fri Sep 24, 2021 6:47 am
-
Isabella Perez Dis3L
- Posts: 100
- Joined: Fri Sep 24, 2021 6:38 am
Re: Sponetanity
Hi, yes we can determine spontaneity from delta G when it is negative, we don't need to look at enthalpy or entropy to determine that. Hope this helps.
Re: Sponetanity
When delta G is positive It's non-spontaneous and negative, the reaction is spontaneous and if positive It's non-spontaneous.
-
sidneypalacios
- Posts: 101
- Joined: Fri Sep 24, 2021 5:30 am
Re: Sponetanity
If all you have is the free Gibbs energy then to my understanding yes you can automatically determine whether it is spontaneous or not. This occurs when ΔG is negative.
-
Samantha Toscano 2C
- Posts: 106
- Joined: Wed Feb 17, 2021 12:24 am
Re: Sponetanity
We can tell that a reaction will be spontaneous solely based on the fact that delta g is negative. Gibbs free energy already takes enthalpy and entropy into account, so if we are given Gibbs free energy we already know.
Re: Sponetanity
Because delta G= deltaH - Tdelta S, we can look only at delta G and if this value is negative, then we can say the reaction is spontaneous.
-
Emily Ang 2B
- Posts: 38
- Joined: Mon Jan 09, 2023 8:52 am
Re: Sponetanity
Just another thing to note about spontaneity is that in these cases spontaneity doesn't mean the reaction will happen out of random, it will just mean that it will happen by itself.
-
Kush Brahmbhatt 2K
- Posts: 38
- Joined: Mon Jan 09, 2023 9:30 am
Re: Sponetanity
The quickest method to determine spontaneity of a reaction is to look at the value of deltaG. If the value of deltaG is negative, then the reaction is considered to be spontaneous (it can occur on its own). You can also determine spontaneity using enthalpy and entropy values through the equation, deltaG = deltaH - T*deltaS.
Return to “Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)”
Who is online
Users browsing this forum: No registered users and 5 guests

