The formula was:
Work Integral Negative? [ENDORSED]
Moderators: Chem_Mod, Chem_Admin
-
Jasmine_Esparza_2A
- Posts: 12
- Joined: Wed Sep 21, 2016 2:58 pm
- Been upvoted: 1 time
Work Integral Negative?
Can someone explain why the formula in lecture for work is negative?
The formula was: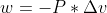
The formula was:
-
Jenny_Luu_2A
- Posts: 20
- Joined: Wed Sep 21, 2016 2:58 pm
Re: Work Integral Negative?
When the system is compressed, the formula has a negative sign and when the system is expanded it has a positive sign.
-
Brianna Wummer 2L
- Posts: 19
- Joined: Wed Sep 21, 2016 2:56 pm
Re: Work Integral Negative? [ENDORSED]
Hey there! 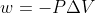 is the energy used as work of expansion equation. This means that this equation represents work for the expansion of a system (NOT its surroundings). Using that equation, when an expansion occurs, there is an increase in volume (positive Delta V) and the system is losing energy, so the work value will be negative. When a compression occurs, there is a decrease in volume (negative Delta V) and the system will gain energy, so the work value will be positive. Without the negative sign in the formula, the above would not be true. Hope that helps!
is the energy used as work of expansion equation. This means that this equation represents work for the expansion of a system (NOT its surroundings). Using that equation, when an expansion occurs, there is an increase in volume (positive Delta V) and the system is losing energy, so the work value will be negative. When a compression occurs, there is a decrease in volume (negative Delta V) and the system will gain energy, so the work value will be positive. Without the negative sign in the formula, the above would not be true. Hope that helps!
Who is online
Users browsing this forum: No registered users and 21 guests

