From 2007 Midterm:
Given that a a gas sample expands, doing 536 kJ of work, while 214 kJ of heat is added to the gas:
a) Calculate DeltaU
b) Will the pressure of the gas be higher or lower when these changes are completed?
I understand Part A:
a) DeltaU = q + w. q is +214 kJ since the heat is ADDED to the gas. w is -536 kJ because the gas is DOING the work and EXPANDING. So DeltaU = 214kJ - 536 kJ. Therefore, DeltaU = -322 kJ.
However, I do not understand Part B. I know that the internal energy change is negative. What is the relationship between DeltaU and Pressure? I can think of PV = nRT. The moles are constant and R is a constant. How can I say that P changes, and in which direction?
Thank you.
Given q and w, will a gas's pressure increase or decrease? [ENDORSED]
Moderators: Chem_Mod, Chem_Admin
-
Michael_Johanis_2K
- Posts: 26
- Joined: Thu Jul 28, 2016 3:00 am
- Been upvoted: 1 time
-
Vivian Wang 3J
- Posts: 29
- Joined: Wed Sep 21, 2016 2:57 pm
- Been upvoted: 1 time
Re: Given q and w, will a gas's pressure increase or decrease?
It is reasonable to say that the pressure is decreasing because the gas is expanding.
-
Michael_Johanis_2K
- Posts: 26
- Joined: Thu Jul 28, 2016 3:00 am
- Been upvoted: 1 time
Re: Given q and w, will a gas's pressure increase or decrease?
Are DeltaU and q not important? Does it only matter that w is negative (gas expands)?
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: Given q and w, will a gas's pressure increase or decrease? [ENDORSED]
If q = w, then 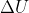 = 0. When
= 0. When 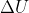 = 0, there is no change in temperature. However, since q < w, the temperature of the gas decreased. Using the relation PV=nRT, since the volume is increasing (expansion) and the temperature must be decreasing (q < w), in order for both sides of the equation to be equal, the pressure must decrease. Therefore, the pressure of the gas will be lower.
= 0, there is no change in temperature. However, since q < w, the temperature of the gas decreased. Using the relation PV=nRT, since the volume is increasing (expansion) and the temperature must be decreasing (q < w), in order for both sides of the equation to be equal, the pressure must decrease. Therefore, the pressure of the gas will be lower.
-
Michael_Johanis_2K
- Posts: 26
- Joined: Thu Jul 28, 2016 3:00 am
- Been upvoted: 1 time
Re: Given q and w, will a gas's pressure increase or decrease?
Thank you! But what if q = -501 kJ and w = 500 kJ (compression)?
Delta U = -1 kJ
PV = nRT
The temperature decreases since DeltaU = negative. The volume decreased since this is compression.
If T goes down, V goes down, n is constant, and R is constant, what happens to P?
Delta U = -1 kJ
PV = nRT
The temperature decreases since DeltaU = negative. The volume decreased since this is compression.
If T goes down, V goes down, n is constant, and R is constant, what happens to P?
Return to “Concepts & Calculations Using First Law of Thermodynamics”
Who is online
Users browsing this forum: No registered users and 5 guests

