The question asks: "How much heat is required to convert a 42.30g block of ice at -5.042 degrees C into water vapor at 150.35 degrees C?"
I know that I have to factor in the change in enthalpy due to the two phase changes. I converted the grams of H2O to moles and multipled that to the deltaH of fusion as well as vaporization to the kJ absorbed during the phase changes.
Then I used the equation q=nC
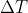
three times (one for each phase since the specific heat changes). I then added all my energy together to get 111.014kJ. However, the answer in the back of the book is 132.0kJ. Help?

