Quiz Preparation
Moderators: Chem_Mod, Chem_Admin
-
Kelly Tran 3N
- Posts: 11
- Joined: Sat Jul 09, 2016 3:00 am
Quiz Preparation
What is the most effective way to prepare for Dr. Lavelle's quizzes? (I had Professor Li for 14A)
-
Natalie_Boyd_1C
- Posts: 14
- Joined: Wed Nov 09, 2016 3:00 am
Re: Quiz Preparation
I've never had him either, but... this might help.
Thus far, the equations we have used are:
enthalpy:
1.definition of enthalpy: delta H= qp=
2. delta H= Hproducts - Hreactants
-->Phase Changes:
3. enthalpy of vaporization: delta Hvaporization= Hvapor - Hliquid
4. enthalpy of fusion: delta Hfusion= Hliquid - Hsolid
5. enthalpy of sublimation: delta Hsublimation=Hvapor - Hsolid
where Hvapor>Hliquid>Hsolid
--> "The three methods"
6. delta Hnet= Hreaction#1 - Hreaction#2
7. delta H= Hbonds broken +
Hbonds broken +  bond formed (bonds formed are negative, btw)
bond formed (bonds formed are negative, btw)
8.=\sum \Delta H^{_{f}^{0}}(products) - \sum \Delta H^{_{f}^{0}}(reactants))
9. delta H=nCpdeltaT
10. w=-PdeltaV
internal energy:
11. delta U =Ufinal-Uinitial
12. V constant delta V=qv
13. delta U= q + w
14. delta U = delta H - PdeltaV
entropy:
15. S=kkln(W)
16. S=Rln(2)
17. S=kBlnW1W2
18. delta S= nRln (V2/V1)= q/T
19. delta= qREV
20.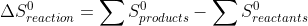
free energy:
21. delta G= delta H -TdeltaS
22. - \sum \Delta G_{f}^{0}(reactants))
23.)
24.)
25.=-\frac{\Delta H^{0}}{RT} + \frac{\Delta S^{0}}{R})
26.=-\frac{\Delta H^{0}}{R}(\frac{1}{T_{2}}-\frac{1}{T_{1}}))
Thus far, the equations we have used are:
enthalpy:
1.definition of enthalpy: delta H= qp=
2. delta H= Hproducts - Hreactants
-->Phase Changes:
3. enthalpy of vaporization: delta Hvaporization= Hvapor - Hliquid
4. enthalpy of fusion: delta Hfusion= Hliquid - Hsolid
5. enthalpy of sublimation: delta Hsublimation=Hvapor - Hsolid
where Hvapor>Hliquid>Hsolid
--> "The three methods"
6. delta Hnet= Hreaction#1 - Hreaction#2
7. delta H=
8.
9. delta H=nCpdeltaT
10. w=-PdeltaV
internal energy:
11. delta U =Ufinal-Uinitial
12. V constant delta V=qv
13. delta U= q + w
14. delta U = delta H - PdeltaV
entropy:
15. S=kkln(W)
16. S=Rln(2)
17. S=kBlnW1W2
18. delta S= nRln (V2/V1)= q/T
19. delta= qREV
20.
free energy:
21. delta G= delta H -TdeltaS
22.
23.
24.
25.
26.
-
Amy_Bugwadia_3I
- Posts: 37
- Joined: Wed Sep 21, 2016 2:56 pm
- Been upvoted: 1 time
Re: Quiz Preparation
The way I prepped for Dr. Lavelle's quizzes last quarter was to first go through the course reader and notes, writing down all important equations/formulas/definitions, just as the previous poster did. I would then do homework problems, noting down which topics were of difficulty for me. I'd next look up those topics in the notes/course reader again, and if that didn't provide enough clarification, I would read that section of the textbook. Finally, I would do the practice quiz (found in the back of the course reader). The UA and TA office hours are also a huge help if you're having difficulty with a certain concept or just want to get more practice.
-
Kristen_Power_1C
- Posts: 13
- Joined: Wed Nov 18, 2015 3:00 am
Re: Quiz Preparation
Hi!
Along with the excellent suggestions of the people above me, I found it helpful to go through the examples and self-checks throughout the chapters that we covered (8 and 9). The examples help with learning how to nail when to use a specific equation, while the self-checks are helpful in thinking in a more "big picture" way about the concepts.
Along with the excellent suggestions of the people above me, I found it helpful to go through the examples and self-checks throughout the chapters that we covered (8 and 9). The examples help with learning how to nail when to use a specific equation, while the self-checks are helpful in thinking in a more "big picture" way about the concepts.
Who is online
Users browsing this forum: No registered users and 4 guests

