CH.1 #33 pt B
Moderators: Chem_Mod, Chem_Admin
-
mendozayael_2H
- Posts: 31
- Joined: Fri Sep 29, 2017 7:06 am
CH.1 #33 pt B
#1.33 says: "The velocity of an electron that is emitted from a metallic surface by a photon is 3.6 X 10^3 km/s. (a) What is the wavelength of the ejected electron? (b) No electrons are emitted from the surface of the metal until the frequency of the radiation reaches 2.50 X 10^16 Hz. How much energy is required to remove the electron from the metal surface?"
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
-
Elizabeth Bamishaye 2I
- Posts: 54
- Joined: Fri Sep 29, 2017 7:04 am
Re: CH.1 #33 pt B
For part A, you determine the mass of electrons, which is 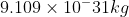 . Then you change the given velocity from kilometers to meters and after that use the equation
. Then you change the given velocity from kilometers to meters and after that use the equation ^{-1}) or basically
or basically 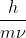 to get the answer for part A.
to get the answer for part A.
For part b, you use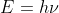 to get the answer for part B.
to get the answer for part B.
For part b, you use
-
Clara Hu 1G
- Posts: 50
- Joined: Sat Jul 22, 2017 3:00 am
- Been upvoted: 1 time
Re: CH.1 #33 pt B
For part b, use 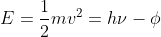 . Because you know the velocity of the ejected electron and the frequency of the radiation, you can solve for the work function, which is the energy required to remove the electron, and get 1.66 x 10-17 J.
. Because you know the velocity of the ejected electron and the frequency of the radiation, you can solve for the work function, which is the energy required to remove the electron, and get 1.66 x 10-17 J.
Return to “Photoelectric Effect”
Who is online
Users browsing this forum: No registered users and 5 guests

