I know we haven't gone over this in class yet, but I'm trying to figure this out. Can someone explain to me why this is statement is true?
"You should expect the uncertainty in the position of an object as heavy as a marble to be very small, but the uncertainty in the speed of an electron, which has a very small mass and is con ned to a small region, can be expected to be very large."
Heisenberg Uncertainty Equation
Moderators: Chem_Mod, Chem_Admin
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: Heisenberg Uncertainty Equation
The Heisenberg Uncertainty Principle is given by the mathematical equation 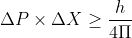
Solving for the uncertainty of the marble's position we get a value very close to zero and solving for the uncertainty of an electrons position, which ways significantly less we get a much larger value. This tells us that it is more difficult to be certain about the position of an electron compared to that of a marble, which makes sense because an electron occupies a given orbital and can be though of as being everywhere in that orbital at once.
Solving for the uncertainty of the marble's position we get a value very close to zero and solving for the uncertainty of an electrons position, which ways significantly less we get a much larger value. This tells us that it is more difficult to be certain about the position of an electron compared to that of a marble, which makes sense because an electron occupies a given orbital and can be though of as being everywhere in that orbital at once.
-
Joshua Xian 1D
- Posts: 51
- Joined: Fri Sep 29, 2017 7:04 am
- Been upvoted: 1 time
Re: Heisenberg Uncertainty Equation
Where does the 4π come from? (Why is it 4π and not some other number?)
Re: Heisenberg Uncertainty Equation
Well, 4pi must have come from some kind of experiment or something. For now, I don't think we are able to understand how scientists derived the specific function, but maybe we will, in the future.
-
Daniel Rivas 3L
- Posts: 32
- Joined: Fri Sep 29, 2017 7:07 am
Re: Heisenberg Uncertainty Equation
Yeah I was wondering about this too and though that maybe I was missing something important. But I guess it isn't something I can realize yet but maybe in the future I will.
-
Rana YT 2L
- Posts: 49
- Joined: Thu Jul 27, 2017 3:01 am
Re: Heisenberg Uncertainty Equation
You can expect the uncertainty to be greater for an electron because an electron has a smaller mass, and since mass is inversely proportional to velocity, would have a greater speed and momentum in comparison to the marble
-
leilawilliams16
- Posts: 20
- Joined: Fri Sep 29, 2017 7:04 am
Re: Heisenberg Uncertainty Equation
can someone provide an example of how this principle is applied in an actual problem? I understand the concept but I haven't really understood how it translates to a real situation
-
Isabelle Bautista 3H
- Posts: 23
- Joined: Fri Sep 29, 2017 7:06 am
Re: Heisenberg Uncertainty Equation
For an example of a real problem using this equation, 1.43 in the book is just that. Exactly the same in concept as the one that was the example in lecture.
Return to “Heisenberg Indeterminacy (Uncertainty) Equation”
Who is online
Users browsing this forum: No registered users and 7 guests

