In 12.21, how does Kw = [H3O+][OH-] manage to turn into [OH-] = Kw/[H3O+]?
Also, are we expected to memorize that Kw = [H3O+][OH-] = 1.0 x 10^-14?
12.21
Acidity 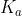
Basicity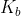
The Conjugate Seesaw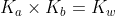
Basicity
The Conjugate Seesaw
Moderators: Chem_Mod, Chem_Admin
-
Mike Vinci 2B
- Posts: 49
- Joined: Fri Sep 29, 2017 7:03 am
- Been upvoted: 1 time
Re: 12.21
Postby Mike Vinci 2B » Wed Dec 06, 2017 11:26 pm
Hi Connie,
Kw is the ionic product of water and is a constant. I'm not sure if we're required to memorize it (it could be on the equations and constants sheet), however I'd double check with a moderator on this forum. In regards to your question, the equation of this formula Kw = [H3O+][OH-] can be solved with algebra by dividing both sides by [H3O+] to get [OH-]=Kw/[H3O+]. The remainder of the problem is a matter of plugging in given information which is 1.0x10^-14 for Kw(it's always this) and the molarity of [H30+] which is also given. By dividing Kw/[H30+], you will get the concentration or molarity of OH-.
Kw is the ionic product of water and is a constant. I'm not sure if we're required to memorize it (it could be on the equations and constants sheet), however I'd double check with a moderator on this forum. In regards to your question, the equation of this formula Kw = [H3O+][OH-] can be solved with algebra by dividing both sides by [H3O+] to get [OH-]=Kw/[H3O+]. The remainder of the problem is a matter of plugging in given information which is 1.0x10^-14 for Kw(it's always this) and the molarity of [H30+] which is also given. By dividing Kw/[H30+], you will get the concentration or molarity of OH-.
Return to “Acidity & Basicity Constants and The Conjugate Seesaw”
Jump to
- NEWS
- NEWS & RESOURCES
- About The Forum
- Forum Rules and Helpful Hints
- How to make a New Post (submit a question) and use Equation Editor (click for details)
- Email Notification (click for details)
- How to Subscribe to a Forum, Subscribe to a Topic, and Bookmark a Topic (click for details)
- Endorsed Post (click for details)
- Multimedia Attachments (click for details)
- Strikethrough (click for details)
- Chem 14A
- Review of Chemical & Physical Principles
- SI Units, Unit Conversions
- Significant Figures
- Accuracy, Precision, Mole, Other Definitions
- Molarity, Solutions, Dilutions
- Empirical & Molecular Formulas
- Balancing Chemical Reactions
- Limiting Reactant Calculations
- The Quantum World
- Properties of Light
- Properties of Electrons
- Einstein Equation
- *Black Body Radiation
- Photoelectric Effect
- Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- DeBroglie Equation
- Heisenberg Indeterminacy (Uncertainty) Equation
- *Shrodinger Equation
- *Particle in a Box
- Wave Functions and s-, p-, d-, f- Orbitals
- Quantum Numbers and The H-Atom
- Electron Configurations for Multi-Electron Atoms
- Trends in The Periodic Table
- Chemical Bonds
- Ionic & Covalent Bonds
- Sigma & Pi Bonds
- Lewis Structures
- Resonance Structures
- Formal Charge and Oxidation Numbers
- Octet Exceptions
- Coordinate Covalent Bonds
- Polarisability of Anions, The Polarizing Power of Cations
- Electronegativity
- Dipole Moments
- Bond Lengths & Energies
- Forces and Liquid Structure
- Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- *Liquid Structure (Viscosity, Surface Tension, Liquid Crystals, Ionic Liquids)
- Molecular Shape and Structure
- Determining Molecular Shape (VSEPR)
- Hybridization
- *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Coordination Compounds and their Biological Importance
- Naming
- Shape, Structure, Coordination Number, Ligands
- Biological Examples
- Industrial Examples
- *Stereochemistry
- *Crystal Field Theory
- *Molecular Orbital Theory Applied To Transition Metals
- Acids and Bases
- Properties & Structures of Inorganic & Organic Acids
- Properties & Structures of Inorganic & Organic Bases
- Amphoteric Compounds
- Lewis Acids & Bases
- Bronsted Acids & Bases
- Conjugate Acids & Bases
- Acidity & Basicity Constants and The Conjugate Seesaw
- Calculating pH or pOH for Strong & Weak Acids & Bases
- Polyprotic Acids & Bases
- Identifying Acidic & Basic Salts
- Calculating the pH of Salt Solutions
- Air Pollution & Acid Rain
- Chem 14A Uploaded Files (Worksheets, etc.)
- Student Social/Study Group
- Administrative Questions and Class Announcements
- General Science Questions
- *Aqueous Equilibria
- *Making Buffers & Calculating Buffer pH (Henderson-Hasselbalch Equation)
- *Biological Importance of Buffer Solutions
- *Titrations & Titration Calculations
- *Indicators
- Chem 14B
- Chemical Equilibrium
- Ideal Gases
- Equilibrium Constants & Calculating Concentrations
- Non-Equilibrium Conditions & The Reaction Quotient
- Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Thermochemistry
- Phase Changes & Related Calculations
- Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Heat Capacities, Calorimeters & Calorimetry Calculations
- Thermodynamics
- Thermodynamic Systems (Open, Closed, Isolated)
- Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Calculating Work of Expansion
- Concepts & Calculations Using First Law of Thermodynamics
- Concepts & Calculations Using Second Law of Thermodynamics
- Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Entropy Changes Due to Changes in Volume and Temperature
- Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Gibbs Free Energy Concepts and Calculations
- Van't Hoff Equation
- Environment, Fossil Fuels, Alternative Fuels
- Biological Examples (*DNA Structural Transitions, etc.)
- Electrochemistry
- Balancing Redox Reactions
- Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Work, Gibbs Free Energy, Cell (Redox) Potentials
- Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Interesting Applications: Rechargeable Batteries (Cell Phones, Notebooks, Cars), Fuel Cells (Space Shuttle), Photovoltaic Cells (Solar Panels), Electrolysis, Rust
- Chemical Kinetics
- Kinetics vs. Thermodynamics Controlling a Reaction
- General Rate Laws
- Method of Initial Rates (To Determine n and k)
- Zero Order Reactions
- First Order Reactions
- Second Order Reactions
- Reaction Mechanisms, Reaction Profiles
- Arrhenius Equation, Activation Energies, Catalysts
- *Enzyme Kinetics
- Experimental Details
- Environment, Ozone, CFCs
- Biological Examples
- Chem 14B Uploaded Files (Worksheets, etc.)
- Student Social/Study Group
- Administrative Questions and Class Announcements
- General Science Questions
- *Thermodynamics and Kinetics of Organic Reactions
- *Electrophiles
- *Nucleophiles
- *Organic Reaction Mechanisms in General
- *Electrophilic Addition
- *Nucleophilic Substitution
- *Free Energy of Activation vs Activation Energy
- *Complex Reaction Coordinate Diagrams
- *Names and Structures of Organic Molecules
- *Alkanes
- *Cycloalkanes
- *Alkenes
- *Cycloalkenes
- *Alkynes
- *Constitutional and Geometric Isomers (cis, Z and trans, E)
- *Haloalkanes
- *Haloalkenes
- *Alcohols
- *Ethers
- *Aldehydes
- *Ketones
- *Carboxylic Acids
- *Amines
- *Identifying Primary, Secondary, Tertiary, Quaternary Carbons, Hydrogens, Nitrogens
- *Conformations of Organic Molecules
- *Alkanes and Substituted Alkanes (Staggered, Eclipsed, Gauche, Anti, Newman Projections)
- *Cyclopropanes and Cyclobutanes
- *Cyclopentanes
- *Cyclohexanes (Chair, Boat, Geometric Isomers)
- *Calculations Using ΔG° = -RT ln K
- *ChemDraw
- *Chem3D
- Chem 14C/D Topics
- Resonance in Organic Compounds
- Stereochemistry in Organic Compounds (Chirality, Stereoisomers, R/S, d/l, Fischer Projections)
Who is online
Users browsing this forum: No registered users and 5 guests

