ICE table
Moderators: Chem_Mod, Chem_Admin
-
Miranda 1J
- Posts: 51
- Joined: Fri Sep 29, 2017 7:06 am
-
nickjadidian 1A
- Posts: 20
- Joined: Fri Sep 29, 2017 7:04 am
Re: ICE table
If x is zero, it means that there is no change in the concentrations of the products or reactants. In this case, x will be zero for both sides. This also implies that the forward and backward reactions have already achieved dynamic equilibrium. The whole point of the ice table is to determine the equilibrium concentrations of all the products and reactants. When x is zero, the chemical concentrations given in the problem are the equilibrium concentrations of the chemical equation.
-
JennyCKim1J
- Posts: 51
- Joined: Fri Sep 29, 2017 7:04 am
-
Scott Chin_1E
- Posts: 55
- Joined: Sat Jul 22, 2017 3:00 am
Re: ICE table
Well we can't assume x=0 because then that would mean there was no change and we know that in a weak acid/base reaction, equilibrium is in constant motion. Instead, we assume x is a very small number, so much so that subtracting it from the initial concentration is negligible and wouldn't affect the overall initial concentration.
For example, if the initial concentration is 0.15M, subtracting that number by 0.000000000000001 would not make a difference to 0.15 at all, thus we can omit x when subtracting it from the initial concentration.
A general rule of thumb is that for any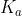 or
or 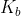 that is less than
that is less than 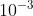 we can assume that the change in concentration for the initial concentration given will be so small, it will not affect the overall answer at all.
we can assume that the change in concentration for the initial concentration given will be so small, it will not affect the overall answer at all.
For example, if the initial concentration is 0.15M, subtracting that number by 0.000000000000001 would not make a difference to 0.15 at all, thus we can omit x when subtracting it from the initial concentration.
A general rule of thumb is that for any
-
Vincent Kim 2I
- Posts: 50
- Joined: Fri Sep 29, 2017 7:04 am
- Been upvoted: 2 times
Re: ICE table
Basically when the Q and K values in the problem are very close, then the changes in concentration (x) will be extremely small, even negligible in most cases.
-
Clarissa Molina 1D
- Posts: 55
- Joined: Fri Sep 29, 2017 7:04 am
Re: ICE table
Can somebody explain the 5% rule when approximating x please? Is K being less than 10^-3 enough to be able to approximate or does it also have to follow the 5% rule?
-
Angel R Morales Dis1G
- Posts: 50
- Joined: Sat Jul 22, 2017 3:01 am
Re: ICE table
Clarissa Molina 1C wrote:Can somebody explain the 5% rule when approximating x please? Is K being less than 10^-3 enough to be able to approximate or does it also have to follow the 5% rule?
The 5% rule and K being less than 10^-3 go hand in hand. The concentrations of the acids/bases are so little, that it should end up being less than 5% the initial concentration. So yeah, K being less than 1.00 x 10-3 is reason enough to approximate.
-
Guadalupe T 1E
- Posts: 58
- Joined: Fri Sep 29, 2017 7:04 am
Re: ICE table
Clarissa Molina 1C wrote:Can somebody explain the 5% rule when approximating x please? Is K being less than 10^-3 enough to be able to approximate or does it also have to follow the 5% rule?
It was mentioned that 10^-3 should be enough to use approximation but you can be asked to prove that it follows the 5% rule.
Return to “Equilibrium Constants & Calculating Concentrations”
Who is online
Users browsing this forum: No registered users and 8 guests

