Molar heat capacity
Moderators: Chem_Mod, Chem_Admin
-
Melodie Oh 2A
- Posts: 22
- Joined: Fri Sep 29, 2017 7:06 am
Molar heat capacity
I'm still confused about the molar heat capacity. How can the specific heat capacity for gas change depending on the value of volume or pressure?
-
David Minasyan 1C
- Posts: 54
- Joined: Thu Jul 13, 2017 3:00 am
Re: Molar heat capacity
From what I know, specific heat is dependent on a unit mass to heat something up. So in terms of a gas, the volume and pressure are important because the smaller the volume, the less molecules of gas and when you heat it up, it would take more heat (thus a higher heat capacity).
-
Max Mazo 2C
- Posts: 30
- Joined: Fri Sep 29, 2017 7:06 am
Re: Molar heat capacity
Using the ideal gas equation, 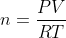 , so the number of moles of a certain gas can change as pressure or volume change.
, so the number of moles of a certain gas can change as pressure or volume change.
-
RohanGupta1G
- Posts: 34
- Joined: Sat Jul 22, 2017 3:00 am
Re: Molar heat capacity
Not sure if this is right or not but the way I was thinking about it was the lower he pressure, the easier it is to add heat and therefore the lower the heat capacity... similarly the greater the volume, the more energy it can absorb and therefore the specific heat capacity is lower... again I’m not sure if this is right or now
Return to “Heat Capacities, Calorimeters & Calorimetry Calculations”
Who is online
Users browsing this forum: No registered users and 6 guests

