Irreversible vs. Reversible [ENDORSED]
Moderators: Chem_Mod, Chem_Admin
Irreversible vs. Reversible
I read about this in the textbook and I understand that you are supposed to use two different equations for each but what is the difference between these two processes?
-
Vasiliki G Dis1C
- Posts: 53
- Joined: Fri Sep 29, 2017 7:04 am
Re: Irreversible vs. Reversible
A reversible process is one that can be reversed by an infinitely small change in a variable. An irreversible process is one where a infinitely small change does not reverse the process of whatever is occurring. Page 265 in the textbook has more information and some good examples. Hope this helps!
-
Joshua Hughes 1L
- Posts: 81
- Joined: Sat Jul 22, 2017 3:01 am
- Been upvoted: 3 times
Re: Irreversible vs. Reversible
Are you just looking for how they differ in their definitions? otherwise I'm not sure I entirely understand. A reversible process is one whose direction can be "reversed" ( the system can be restored to the initial state from the final state) by inducing infinitesimal changes to some property of the system(ie temp, pressure or something like that).
Irreversible ones are ones that occur mostly naturally in nature I think. Wikipedia defines them as this:
"In thermodynamics, a change in the thermodynamic state of a system and all of its surroundings cannot be precisely restored to its initial state by infinitesimal changes in some property of the system without expenditure of energy. A system that undergoes an irreversible process may still be capable of returning to its initial state. However, the impossibility occurs in restoring the environment to its own initial conditions. An irreversible process increases the entropy of the universe"
The book gives an example of the piston moving based on the outside pressure and whether infinitesimal changes to the pressure would cause a movement in the piston.
Irreversible ones are ones that occur mostly naturally in nature I think. Wikipedia defines them as this:
"In thermodynamics, a change in the thermodynamic state of a system and all of its surroundings cannot be precisely restored to its initial state by infinitesimal changes in some property of the system without expenditure of energy. A system that undergoes an irreversible process may still be capable of returning to its initial state. However, the impossibility occurs in restoring the environment to its own initial conditions. An irreversible process increases the entropy of the universe"
The book gives an example of the piston moving based on the outside pressure and whether infinitesimal changes to the pressure would cause a movement in the piston.
-
Joshua Hughes 1L
- Posts: 81
- Joined: Sat Jul 22, 2017 3:01 am
- Been upvoted: 3 times
Re: Irreversible vs. Reversible
OOps sorry, I didnt see someone else posted.
You can also look up some websites that explain stuff a bit more in detail or provide more examples than the book.
Try this web page:
http://www.brighthubengineering.com/the ... processes/
You can also look up some websites that explain stuff a bit more in detail or provide more examples than the book.
Try this web page:
http://www.brighthubengineering.com/the ... processes/
-
melissa carey 1f
- Posts: 53
- Joined: Fri Sep 29, 2017 7:06 am
Re: Irreversible vs. Reversible
To clarify: will irreversible expansion be in test #1? I thought Dr. Lavelle said that it would not be tested, but it appears in the assigned homework problems? Thanks!
-
melissa carey 1f
- Posts: 53
- Joined: Fri Sep 29, 2017 7:06 am
Re: Irreversible vs. Reversible
To clarify: will irreversible expansion be in test #1? I thought Dr. Lavelle said that it would not be tested, but it appears in the assigned homework problems? Thanks!
-
Erica Nagase 1H
- Posts: 30
- Joined: Sat Jul 22, 2017 3:00 am
Re: Irreversible vs. Reversible
In a reversible pathway, pressure is changing and more work is done (w=-nRTln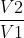 ). In an irreversible pathway, pressure is constant and less work is done (w=-p
). In an irreversible pathway, pressure is constant and less work is done (w=-p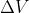 ).
).
-
Mika Sonnleitner 1A
- Posts: 50
- Joined: Fri Sep 29, 2017 7:04 am
- Been upvoted: 2 times
Re: Irreversible vs. Reversible
Additionally, reversible systems involved, for example, a bicycle pump with equal pressure inside and outside of the pump. An irreversible system, however, would have differing pressure inside and outside of the bicycle pump example. However, all real/biological processes are highly irreversible, in order to speed up the reactions. These irreversible reactions are also less efficient than reversible reactions, because there is less useful work done.
-
siannehazel1B
- Posts: 30
- Joined: Fri Sep 29, 2017 7:06 am
Re: Irreversible vs. Reversible
how does the equation w=-nRTlnV2/V1 account for the changing pressure and more work being done as opposed to the equation for the irreversible pathway?
Re: Irreversible vs. Reversible
The reversible equation is derived from the differential equation for work that uses incremental volume or changes to estimate maximum theoretical work, i.e. how much work would be done if the entirety of heat (q) powers work (w)
-
Alexia Joseph 2B
- Posts: 56
- Joined: Thu Jul 27, 2017 3:01 am
Re: Irreversible vs. Reversible [ENDORSED]
When you think of an irreversible or reversible process, think in terms of the piston and external pressure example. In one, the system had 2 atm and the Pexternal was 2 atm. In the other, the system had 2 atm and the Pexternal was 1 atm. In the first example, the pressures are at thermal equilibrium, and the system will be reversible (implies more work done in the system because the system is pushing against an equal/higher Pexternal. In the second example, the system is irreversible because it is pushing against a lower Pexternal and doing less work.
Return to “Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)”
Who is online
Users browsing this forum: No registered users and 6 guests

