Enthalpy of Reaction at lower temperatures [ENDORSED]
Moderators: Chem_Mod, Chem_Admin
-
Carlos Gonzales 1H
- Posts: 50
- Joined: Fri Sep 29, 2017 7:05 am
- Been upvoted: 1 time
Enthalpy of Reaction at lower temperatures
Could someone please explain a little more why enthalpy of reaction has a bigger role at lower T? Thanks.
-
Rachel Formaker 1E
- Posts: 86
- Joined: Fri Sep 29, 2017 7:04 am
- Been upvoted: 2 times
Re: Enthalpy of Reaction at lower temperatures
At lower temperatures, enthalpy of a reaction (ΔH or qP) has a bigger influence on the entropy of a reaction because of this equation:
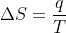
When T is lower, ΔS is larger, meaning that q had a larger effect on entropy.
For example, if q=10 and T=1, ΔS =10, but if q=10 and T=10, then ΔS=1
So a lower temperature (T) means that the heat transferred (q) has a greater impact on entropy (ΔS).
When T is lower, ΔS is larger, meaning that q had a larger effect on entropy.
For example, if q=10 and T=1, ΔS =10, but if q=10 and T=10, then ΔS=1
So a lower temperature (T) means that the heat transferred (q) has a greater impact on entropy (ΔS).
-
Juanyi Tan 2K
- Posts: 33
- Joined: Fri Sep 29, 2017 7:05 am
Re: Enthalpy of Reaction at lower temperatures [ENDORSED]
I find an explanation in the textbook to be helpful: just imagine how sneezing in a quiet environment will attract attention more than in a noisy environment. The energy transfer to a cool system where there is little thermal motion will result in a more noticeable change, leading to a greater entropy.
Who is online
Users browsing this forum: No registered users and 4 guests

