degeneracy [ENDORSED]
Moderators: Chem_Mod, Chem_Admin
-
Phi Phi Do 2E
- Posts: 27
- Joined: Fri Sep 29, 2017 7:05 am
degeneracy
Can someone please explain more about the concept of degeneracy and how that relates to entropy?
-
Victoria Draper 1G
- Posts: 52
- Joined: Fri Sep 29, 2017 7:05 am
- Been upvoted: 1 time
Re: degeneracy
Degeneracy is the number of different ways energy can exist. Degeneracy and entropy are directly related and therefore the higher the entropy the more number of ways energy can exist which means a higher degeneracy.
-
Miranda 1J
- Posts: 51
- Joined: Fri Sep 29, 2017 7:06 am
Re: degeneracy
Degeneracy is basically the number of different ways energy can exist and the higher the entropy then the more the ways that energy can exist (proportional).
-
Julian Krzysiak 2K
- Posts: 49
- Joined: Fri Sep 29, 2017 7:07 am
Re: degeneracy
Degeneracy is the total number of ways of achieving a given energy state.
In the dogbone example Dr. Lavelle showed us, we could could calculate W with the info given by . N is the number of particles, molecules, etc., while the 2 was the number of equivalent states. So if there was 1 particle w/ 2 equivalent states,
. N is the number of particles, molecules, etc., while the 2 was the number of equivalent states. So if there was 1 particle w/ 2 equivalent states, 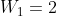 , as 1 particle can only have 2 possible positions here.
, as 1 particle can only have 2 possible positions here.
But with the 4 dogbones, we were given 2 particles w/ 2 equivalent states, , because the particles could be in positions A--B, B--A, AB--, --AB.
, because the particles could be in positions A--B, B--A, AB--, --AB.
They are related directly via the Boltzman Equation,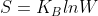
As the degeneracy increases, entropy will also increase.
In the dogbone example Dr. Lavelle showed us, we could could calculate W with the info given by
But with the 4 dogbones, we were given 2 particles w/ 2 equivalent states,
They are related directly via the Boltzman Equation,
As the degeneracy increases, entropy will also increase.
-
Glendy Gonzalez 1A
- Posts: 52
- Joined: Fri Sep 29, 2017 7:07 am
Re: degeneracy
Degeneracy refers to the number of ways of obtaining a certain energy state. Degeneracy and entropy are inverses of each other. That is, the higher the degeneracy, the lower the entropy.
-
Belle Calforda3f
- Posts: 67
- Joined: Fri Sep 29, 2017 7:07 am
-
AnuPanneerselvam1H
- Posts: 52
- Joined: Fri Sep 29, 2017 7:07 am
Re: degeneracy [ENDORSED]
Degeneracy is the number of ways of achieving a given energy state. An example given in class was that a quarter has a degeneracy of 2 since it can either land on heads or tails. The higher the entropy, the higher amount of possible energy states.
Return to “Phase Changes & Related Calculations”
Who is online
Users browsing this forum: No registered users and 7 guests

