Entropy and Temperature
Moderators: Chem_Mod, Chem_Admin
Entropy and Temperature
I understand that, according to 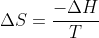 , temperature and entropy are inversely related. So, an increase in temperature would result in a decrease in entropy. But I don't understand that because, how I see it, and increase in temperature results in an increase in the speed of the molecules, which results in an increase in entropy. And that goes against what the equation says. If anyone could please clarify my misconception, I'd appreciate it.
, temperature and entropy are inversely related. So, an increase in temperature would result in a decrease in entropy. But I don't understand that because, how I see it, and increase in temperature results in an increase in the speed of the molecules, which results in an increase in entropy. And that goes against what the equation says. If anyone could please clarify my misconception, I'd appreciate it.
-
Sabrina Dunbar 1I
- Posts: 57
- Joined: Fri Sep 29, 2017 7:07 am
- Been upvoted: 1 time
Re: Entropy and Temperature
The term ∆S means the change in entropy, not just entropy itself. By increasing the temperature, the change in entropy is less than if the reaction were to occur at a lower temperature. The larger T value in the denominator makes this ∆S value less because as you said, the two are inversely related. Hopefully this clarifies enough of your questions:)
-
Eli Aminpour 2K
- Posts: 50
- Joined: Fri Sep 29, 2017 7:04 am
Re: Entropy and Temperature
Also, we used this equation in class to find the change in entropy when temperature is constant (as ice melts for example). If you want to find the change in entropy when temperature is not constant, use the equation delta S=n*C*ln(t2/t1)
-
Adrian Lim 1G
- Posts: 88
- Joined: Fri Sep 29, 2017 7:03 am
Return to “Concepts & Calculations Using Second Law of Thermodynamics”
Who is online
Users browsing this forum: No registered users and 4 guests

