Delta G not
Moderators: Chem_Mod, Chem_Admin
-
Leah Thomas 2E
- Posts: 51
- Joined: Fri Sep 29, 2017 7:06 am
Delta G not
Can someone explain the difference between ∆G and ΔG° conceptually. Why don't we always need to calculate for ΔG°?
-
Cyianna 2F
- Posts: 34
- Joined: Wed Nov 16, 2016 3:04 am
Re: Delta G not
It's just a difference of values, the concept of 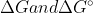 are still the same. I think that it just depends of the conditions of the reaction. I'm not entirely sure though, that's a good question.
are still the same. I think that it just depends of the conditions of the reaction. I'm not entirely sure though, that's a good question.
-
Jason Muljadi 2C
- Posts: 62
- Joined: Thu Jul 27, 2017 3:01 am
Re: Delta G not
ΔG° is in standard condition, so you would have to calculate ΔH° and ΔS°, just like you would in the regular Gibbs Free Energy equation.
-
Jakob von Morgenland 2C
- Posts: 31
- Joined: Sat Jul 22, 2017 3:00 am
- Been upvoted: 1 time
Re: Delta G not
∆G° is considered the standard free energy change of a reaction, while ∆G is considered the free energy change of a reaction. Remember, standard free energy change implies that the reactants and products are in their standard states at 1 atm and generally 25 celsius.
Re: Delta G not
∆G° is the standard free energy of the reaction. This means that the reactants and products are at their standard state: 1 atm for gases and 1 M of aqueous solutions, typically at 25°C. ∆G is just the free energy of the reaction that doesn't have to be under the standard state conditions.
-
Diego Zavala 2I
- Posts: 65
- Joined: Fri Sep 29, 2017 7:07 am
- Been upvoted: 1 time
Re: Delta G not
Return to “Gibbs Free Energy Concepts and Calculations”
Who is online
Users browsing this forum: No registered users and 4 guests

