Temperature and Δ U
Moderators: Chem_Mod, Chem_Admin
-
Leah Thomas 2E
- Posts: 51
- Joined: Fri Sep 29, 2017 7:06 am
Temperature and Δ U
When temperature is constant, why do we assume Δ U=0. I understand that Δ U=q+w and q=0 if temperature is constant but what about work? How does that relate to temperature?
-
Jakob von Morgenland 2C
- Posts: 31
- Joined: Sat Jul 22, 2017 3:00 am
- Been upvoted: 1 time
Re: Temperature and Δ U
If deltaU=0, then deltaU=0=q+w, or q=-w. As you said, q would equal 0 if the temperature is constant, so sub that in for q, and you would get q=0=-w, or w=0. This is assumed for and ideal gas in an isothermal process generally.
-
Luis De La Cruz 1H
- Posts: 37
- Joined: Fri Sep 25, 2015 3:00 am
Re: Temperature and Δ U
The important thing to realize is that when Temperature is constant as is the case in isothermal processes,  , q=0 only in the instance of an adiabatic process, in which no heat is exchanged between the system and its surroundings. To relate work to temperature in the instance of an isothermal process (temperature is constant), such as the isothermal compression of an ideal gas, doing work on the gas increases the internal energy and will tend to increase the temperature. Therefore, to maintain the temperature constant, energy must leave the system as heat and enter the environment, and therefore why
, q=0 only in the instance of an adiabatic process, in which no heat is exchanged between the system and its surroundings. To relate work to temperature in the instance of an isothermal process (temperature is constant), such as the isothermal compression of an ideal gas, doing work on the gas increases the internal energy and will tend to increase the temperature. Therefore, to maintain the temperature constant, energy must leave the system as heat and enter the environment, and therefore why  , instead we use -q=w, which means that the heat leaving the system is equal to the work being done on the system by the compression and thus why we assume
, instead we use -q=w, which means that the heat leaving the system is equal to the work being done on the system by the compression and thus why we assume 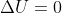 . Because -q=w relationship can only be true when
. Because -q=w relationship can only be true when 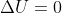 since as you stated
since as you stated 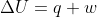 .
.
Return to “Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)”
Who is online
Users browsing this forum: No registered users and 5 guests

