Entropy Equation
Moderators: Chem_Mod, Chem_Admin
-
Lindsay H 2B
- Posts: 50
- Joined: Fri Sep 29, 2017 7:07 am
- Been upvoted: 1 time
Entropy Equation
In my notes from the lecture a few weeks ago on wednesday 1/24 I have written that ∆S=-nRTln(V2/V1) but the solutions manual and the book both use that equation without the negative sign. Can anyone explain why that is?
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: ∆S=nRTln(Vf/Vi)
The equation you wrote down is actually the equation for work. Please see Dr. Lavelle's constants and equations sheet on his website for clarification. https://lavelle.chem.ucla.edu/wp-conten ... ations.pdf
Also note that there is no T variable in the equation for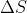 when there is a volume change.
when there is a volume change.
Also note that there is no T variable in the equation for
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: ∆S=nRTln(Vf/Vi)
Your expression is indeed that for work, not change in entropy. But this is a matter of going through the derivation.
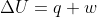
Under an isothermal process,
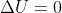
, so that
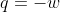
Work under an isothermal reaction is
)
And change in entropy, with constant temperature, is
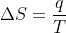
Substitute w for q in the above equation
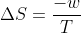
Substitute the expression of W under an isothermal process for
)
The final equation is how you would calculate the change in entropy for an isothermal process.
Under an isothermal process,
, so that
Work under an isothermal reaction is
And change in entropy, with constant temperature, is
Substitute w for q in the above equation
Substitute the expression of W under an isothermal process for
The final equation is how you would calculate the change in entropy for an isothermal process.
Return to “Calculating Work of Expansion”
Who is online
Users browsing this forum: No registered users and 3 guests

