Cv vs. Cp [ENDORSED]
Moderators: Chem_Mod, Chem_Admin
-
Miranda 1J
- Posts: 51
- Joined: Fri Sep 29, 2017 7:06 am
Cv vs. Cp
Can someone please explain when we use Cv and when we use Cp and how we would know which one to use? Also, what's the difference between DH= nCpDT and DH= gCspDT? Does it matter which one we use?
Thanks!
Thanks!
Re: Cv vs. Cp
Use Cv when there's constant volume. This is the specific heat when there's constant volume.
Use Cp when there's constant pressure. This is the specific heat when there's constant pressure.
Use Cp when there's constant pressure. This is the specific heat when there's constant pressure.
-
Clarisse Wikstrom 1H
- Posts: 63
- Joined: Fri Sep 29, 2017 7:05 am
Re: Cv vs. Cp
Cv is the molar heat capacity of a gas @ CONSTANT VOLUME, whereas Cp is the molar heat capacity of a gas at CONSTANT PRESSURE. In one example (#31), you would use it when doing q=mCdeltaT if you're given an ideal gas but no molar heat capacity. The Cp and Cv for ideal gases can be found on Lavelle's equation sheet on his website.
-
Julian Krzysiak 2K
- Posts: 49
- Joined: Fri Sep 29, 2017 7:07 am
Re: Cv vs. Cp
Cp is the heat capacity for something at a constant pressure, and would be equal to 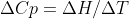
Cv is the heat capacity for something at a constant volume, and would be equal to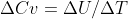
You'll know when to use a particular heat capacity because they'll specify in the problem the conditions of the reaction, or you'll have to infer the conditions, for example, when there is an open container, it will have to be at a constant pressure of 1atm.
You usually use these equations when dealing with anything in calculating how much heat was given off, the change in temperature, or any variation in dealing with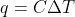
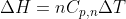 is the specific heat capacity for something at a constant pressure in terms of moles
is the specific heat capacity for something at a constant pressure in terms of moles
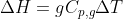 is the specific heat capacity for something at a constant pressure in terms of grams
is the specific heat capacity for something at a constant pressure in terms of grams
So you'll have to use either one depending in which units they give the amount to you, or if given enough information, you could convert grams into moles, vice versa, and then use which equation you want.
Cv is the heat capacity for something at a constant volume, and would be equal to
You'll know when to use a particular heat capacity because they'll specify in the problem the conditions of the reaction, or you'll have to infer the conditions, for example, when there is an open container, it will have to be at a constant pressure of 1atm.
You usually use these equations when dealing with anything in calculating how much heat was given off, the change in temperature, or any variation in dealing with
So you'll have to use either one depending in which units they give the amount to you, or if given enough information, you could convert grams into moles, vice versa, and then use which equation you want.
-
AlyssaPeckham1A
- Posts: 49
- Joined: Fri Sep 29, 2017 7:04 am
Re: Cv vs. Cp
Also to solve for the values of Cvm and Cpm you can use the equations Cvm=3/2*R and Cpm=5/2*R for the molar heat capacity.
-
Katelyn 2E
- Posts: 35
- Joined: Sat Jul 22, 2017 3:01 am
-
Lucia H 2L
- Posts: 43
- Joined: Mon Nov 14, 2016 3:00 am
- Been upvoted: 1 time
Re: Cv vs. Cp
They are listed on the formula sheet!
Ideal gas, Cp = (5/2)R
Ideal gas, Cv = (3/2)R
Both are constant because heat capacity for gases isn't dependent on temp or volume.
Ideal gas, Cp = (5/2)R
Ideal gas, Cv = (3/2)R
Both are constant because heat capacity for gases isn't dependent on temp or volume.
-
Justin Bui 2L
- Posts: 51
- Joined: Fri Sep 29, 2017 7:06 am
Re: Cv vs. Cp [ENDORSED]
We would also be told the conditions of the problem and then we would know which one to use, Cp or Cv, based off of that.
-
Guadalupe T 1E
- Posts: 58
- Joined: Fri Sep 29, 2017 7:04 am
-
Warda Sahib 2J
- Posts: 29
- Joined: Thu Jul 13, 2017 3:00 am
-
Abby Ellstrom 1I
- Posts: 53
- Joined: Fri Sep 29, 2017 7:04 am
-
Brian Chang 2H
- Posts: 65
- Joined: Fri Sep 28, 2018 12:17 am
-
Jeannine 1I
- Posts: 73
- Joined: Fri Sep 28, 2018 12:27 am
Return to “Heat Capacities, Calorimeters & Calorimetry Calculations”
Who is online
Users browsing this forum: No registered users and 6 guests

