Enthalpy of fusion [ENDORSED]
Moderators: Chem_Mod, Chem_Admin
-
Cristian Carrasco 1F
- Posts: 52
- Joined: Fri Sep 29, 2017 7:06 am
Enthalpy of fusion
for problem 2 on the midterm, how would you find the delta H fusion of the ice cream?
-
Nha Dang 2I
- Posts: 53
- Joined: Fri Sep 29, 2017 7:07 am
Re: Enthalpy of fusion
You set it up as q=mcdeltaT + deltaH(fusion)(1/2m), which becomes deltaH(fusion)=((2)(q-mcdeltaT))/(m). Then you just plug in the numbers and you end up getting 3.7 kJ/g.
-
Erica Nagase 1H
- Posts: 30
- Joined: Sat Jul 22, 2017 3:00 am
Re: Enthalpy of fusion [ENDORSED]
The total energy put into the ice cream is equal to the energy needed to raise the temperature from -2.8 to 0degC plus the energy needed to melt it (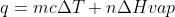 )
)
-
Liz White 1K
- Posts: 35
- Joined: Thu Jul 13, 2017 3:00 am
Re: Enthalpy of fusion
Can someone please explain the answer to this question in more detail? I don't understand why we use q=mcdeltaT + deltaH. Also, I plugged the values into this and got an incorrect answer.
Re: Enthalpy of fusion
We use the equation 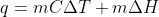 to find the qice cream because we have to account for the fact that it changes from solid (frozen) to liquid (melted). The
to find the qice cream because we have to account for the fact that it changes from solid (frozen) to liquid (melted). The 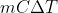 accounts for the ice cream changing and then
accounts for the ice cream changing and then 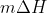 accounts for the phase change, where
accounts for the phase change, where 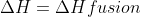 .
.
Return to “Concepts & Calculations Using First Law of Thermodynamics”
Who is online
Users browsing this forum: No registered users and 5 guests

