Unique Rate [ENDORSED]
Moderators: Chem_Mod, Chem_Admin
-
104922499 1F
- Posts: 53
- Joined: Fri Sep 29, 2017 7:04 am
Unique Rate
Can someone clarify what unique rate laws are? I did not understand what Lavelle said during class about this. Thanks!
-
Sabrina Dunbar 1I
- Posts: 57
- Joined: Fri Sep 29, 2017 7:07 am
- Been upvoted: 1 time
Re: Unique Rate
The unique rate is the instantaneous rate of change at any time t during the reaction. Unlike the average rate, which takes into account all of the times at which the reaction proceeds and averages it, the instantaneous rate is specific to a time and therefore it generates a different k value. The general equation used for the unique rate can be written as rate=d[R]/dt while the average rate is rate=∆concentration/∆t. Unique rate laws are specific to each reaction and are confined to a specific time.
-
Alvin Tran 2E
- Posts: 39
- Joined: Fri Sep 29, 2017 7:06 am
Re: Unique Rate
In addition, when you calculate the unique rate, it is the same for all the reactants and products involved in the reaction.
-
Angela G 2K
- Posts: 30
- Joined: Fri Sep 29, 2017 7:06 am
Re: Unique Rate [ENDORSED]
For a reaction aA + bB -> cC + dD, where the lowercase letters are the stoichiometric coefficients and the uppercase letters are chemical species, the unique rate law is
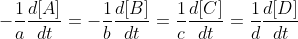
This rate law is unique to the reaction and the same for all reactants and products in this reaction.
This rate law is unique to the reaction and the same for all reactants and products in this reaction.
Who is online
Users browsing this forum: No registered users and 3 guests

