Arrange the following types of photons of electromagnetic radiation in order of increasing energy: gamma rays, x-rays, visible light, ultraviolet radiation, microwaves.
Why do gamma rays have the most amount of energy? If energy is proportional to frequency then how does the wavelength tell us about the energy?
Homework 1.5
Moderators: Chem_Mod, Chem_Admin
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: Homework 1.5
gamma rays have the highest frequency, so by E = hv, its photons should have the highest energy. you can convert wavelength to frequency using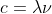
-
Valeria Viera 1B
- Posts: 60
- Joined: Fri Apr 06, 2018 11:05 am
Re: Homework 1.5
gamma rays have a higher energy since they have a higher frequency and a shorter wavelength (it helps to look at pictures of this)
-
Surya Palavali 1D
- Posts: 24
- Joined: Fri Apr 06, 2018 11:04 am
Re: Homework 1.5
A lower wavelength means the frequency is higher because there are more waves in the same amount of time. So, because the frequency of gamma rays is the highest, their energy will also be the highest.
-
joannehaddad
- Posts: 31
- Joined: Fri Apr 06, 2018 11:03 am
Re: Homework 1.5
Keep in mind that the relationship between frequency, energy, and wavelength is as follows:
The longer the wavelength the lower the frequency and that means less energy.
The longer the wavelength the lower the frequency and that means less energy.
-
Jake Gordon 1A
- Posts: 61
- Joined: Fri Sep 28, 2018 12:15 am
Re: Homework 1.5
Gamma rays have the most energy because their frequency is highest. Frequency and energy are directly proportional. Higher frequency means higher energy. Wavelength and Frequency are inversely proportional (this also makes sense if you draw it out, longer distance between crests means less space for the total number of waves) therefore as one increases the other decreases and vice versa. With these relationships you can determine how energy is affected based on changes in wavelength or frequency.
-
Michelle Nwufo 2G
- Posts: 61
- Joined: Fri May 18, 2018 3:00 am
Re: Homework 1.5
Because energy and frequency are proportional, if the question asked to arrange the photons in order of increasing frequency, would the answer remain the same?
Return to “Properties of Light”
Who is online
Users browsing this forum: No registered users and 8 guests

