Schrodinger Equation [ENDORSED]
Moderators: Chem_Mod, Chem_Admin
-
Katarina Ho -1B
- Posts: 29
- Joined: Fri Apr 06, 2018 11:03 am
Schrodinger Equation
I am a little confused about the application to this equation. Is it only used for Hyrogen atoms? or can it be applied to all atoms?
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: Schrodinger Equation
It can be applied to any atom and any system. The orbitals we are familiar with are the solutions when we apply it to hydrogen.
If it is written as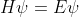 , then
, then 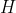 is called an operator (like
is called an operator (like 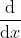 in calculus) and depends on the specific system we are examining. If it is hydrogen, when we solve it we get different functions
in calculus) and depends on the specific system we are examining. If it is hydrogen, when we solve it we get different functions 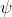 which look like the orbitals, and different values of
which look like the orbitals, and different values of 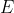 , which are the corresponding energies of the orbitals. For hydrogen, we know that
, which are the corresponding energies of the orbitals. For hydrogen, we know that 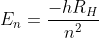 . We got this equation for
. We got this equation for 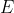 by solving the Schrodinger equation!
by solving the Schrodinger equation!
If it is written as
-
Bree Perkins 1E
- Posts: 34
- Joined: Fri Apr 06, 2018 11:04 am
Re: Schrodinger Equation
What does the 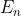 equation mean in this instance. I guess I am confused on what we use the Schrodinger Equation for?
equation mean in this instance. I guess I am confused on what we use the Schrodinger Equation for?
-
Ellie Tsang 1I
- Posts: 30
- Joined: Fri Apr 06, 2018 11:05 am
- Been upvoted: 1 time
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: Schrodinger Equation
The Schroedinger equation is used to derive solutions to quantum mechanical systems. For example, the orbital functions for the hydrogen atom were generated from solutions to the equation. Particle-in-a-box is another system that can be modeled using the equation.
-
Aryana Nazem 1A
- Posts: 40
- Joined: Fri Apr 06, 2018 11:04 am
Re: Schrodinger Equation
Does anyone know if this equation will be fair game for our midterm? Also will we just have to know what it means conceptually or will we have to actually need to use it?
-
Jacy Black 1C
- Posts: 30
- Joined: Fri Apr 06, 2018 11:01 am
Re: Schrodinger Equation
I understand that Schrodinger's equation gives a solution of the wave function which describes orbitals. But how? I'm unsure what the notation itself for the equation means, and how I would describe this system or answer a test question using it.
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: Schrodinger Equation [ENDORSED]
You will be responsible for a conceptual understanding of the equation, similar to the explanations above. However, you will not be expected to use the equation itself on an exam.
-
Elana Weingord 1C
- Posts: 34
- Joined: Fri Apr 06, 2018 11:05 am
Return to “*Shrodinger Equation”
Who is online
Users browsing this forum: No registered users and 8 guests

