In class problem [ENDORSED]
Moderators: Chem_Mod, Chem_Admin
-
Alysia Garcia 1B
- Posts: 40
- Joined: Wed Feb 21, 2018 3:02 am
In class problem
For the problem we did in class, how would we determine if the outcome of uncertainty is an impossible outcome? the answer was 3.4x10^10 m/s.
-
Alejandro Salazar 1D
- Posts: 31
- Joined: Fri Sep 29, 2017 7:05 am
Re: In class problem
I believe the answer has to be smaller than the speed of light 3.00 x10^8 m/s to make sense. In this case, 3.4 x 10^10 m/s is larger than the speed of light, so the object is too large
-
Harmonie Ahuna-1C
- Posts: 30
- Joined: Fri Apr 06, 2018 11:01 am
Re: In class problem
In this case, because we solved for the uncertainty in velocity we could compare the value to the speed of light because nothing can travel faster than it. I'm not sure what we would compare if we were looking at other things like the reasonability of uncertainty in position.
-
Anna De Schutter - 1A
- Posts: 66
- Joined: Wed Feb 21, 2018 3:01 am
Re: In class problem [ENDORSED]
I agree that for the example we did in class, we could say it was an impossible outcome because the the speed of an electron can never be faster than the speed of light since it has a mass.
Maybe to determine if the uncertainty of position is reasonable we could have compared it to the diameter of the nucleus? Like for example if we were given the uncertainty in velocity of the speed of the electron, we could have calculated the uncertainty in position of the electron following the formula:
(uncertainty in momentum)*(uncertainty in position)>=h/(4*pi) where uncertainty in momentum=mass*uncertainty in velocity
And then seen how it compared to the diameter of the nucleus (which is around 10^-15m). If the uncertainty in position was larger than 10^-15m, we would have said it was unreasonable to say the electron is confined to the nucleus (which it is), but if it was smaller than 10^-15m, we could have said it was reasonable (but we know the latter is impossible, the electron isn't confined to the nucleus).
I think, in any situation, if we want to know if the uncertainty of position is reasonable, we can compare it to the dimension that situation confines us too. But I'm not too sure about my answer...
Anna De Schutter, section 1A
Maybe to determine if the uncertainty of position is reasonable we could have compared it to the diameter of the nucleus? Like for example if we were given the uncertainty in velocity of the speed of the electron, we could have calculated the uncertainty in position of the electron following the formula:
(uncertainty in momentum)*(uncertainty in position)>=h/(4*pi) where uncertainty in momentum=mass*uncertainty in velocity
And then seen how it compared to the diameter of the nucleus (which is around 10^-15m). If the uncertainty in position was larger than 10^-15m, we would have said it was unreasonable to say the electron is confined to the nucleus (which it is), but if it was smaller than 10^-15m, we could have said it was reasonable (but we know the latter is impossible, the electron isn't confined to the nucleus).
I think, in any situation, if we want to know if the uncertainty of position is reasonable, we can compare it to the dimension that situation confines us too. But I'm not too sure about my answer...
Anna De Schutter, section 1A
-
Alma Flores 1D
- Posts: 64
- Joined: Wed Nov 08, 2017 3:01 am
Re: In class problem
The uncertainty (3.4x10^10 m/s) is greater than the speed of light (3.0x10^8 m/s). This makes it an unrealistic number.
-
AnnaYan_1l
- Posts: 96
- Joined: Fri Apr 06, 2018 11:05 am
- Been upvoted: 1 time
Re: In class problem
Yes, I agree with the people above. Additionally, in the video module, Professor Lavelle showed the correct atomic model:
The electron is not confined to the nucleus and we now know, from experimental observation that the size of an atom is determined by its electrons outside of the nucleus. For a Hydrogen atom, the electron is confined to its atomic diameter of 2.5 x 10^(-10) m (which would be the electron's uncertainty in position)
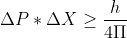
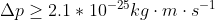
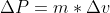
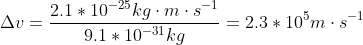
This is a large number but it is physically possible (is below the parameters of the speed of light) so it is a more reasonable number which indicates it's a more stable and realistic model! Hope that helps to clarify the difference between the two (a model that works, and one that doesn't work).
The electron is not confined to the nucleus and we now know, from experimental observation that the size of an atom is determined by its electrons outside of the nucleus. For a Hydrogen atom, the electron is confined to its atomic diameter of 2.5 x 10^(-10) m (which would be the electron's uncertainty in position)
This is a large number but it is physically possible (is below the parameters of the speed of light) so it is a more reasonable number which indicates it's a more stable and realistic model! Hope that helps to clarify the difference between the two (a model that works, and one that doesn't work).
Re: In class problem
light is the fastest speed in the whole universe
according to the General Relativity. If there is something faster than light...then the whole theories of physic will collapse!
so if the value of speed you calculated is faster than 3E8, it's unrealistic
according to the General Relativity. If there is something faster than light...then the whole theories of physic will collapse!
so if the value of speed you calculated is faster than 3E8, it's unrealistic
Return to “Heisenberg Indeterminacy (Uncertainty) Equation”
Who is online
Users browsing this forum: No registered users and 3 guests

