A piece of copper of mass 20.0 g at 100.0 degrees Celsius is placed in a vessel of negligible heat capacity but containing 50.7 g of water at 22.0 degrees Celsius. Calculate the final temperature of water. Assume that no energy is lost to surroundings.
How do I go about solving this problem?
HW Problem 4A.9
Moderators: Chem_Mod, Chem_Admin
-
Vincent Li 4L
- Posts: 48
- Joined: Fri Sep 28, 2018 12:19 am
Re: HW Problem 4A.9
For this problem, think about where the heat is going. Because there is a temperature difference, energy will be transferred from the copper and into the water. Since heat released = - heat absorbed, we can use q = mC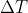 for both substances, and we solve for Tfinal since we should know all other values. Hope this helps.
for both substances, and we solve for Tfinal since we should know all other values. Hope this helps.
-
Tuong-Minh Tran 1C
- Posts: 30
- Joined: Fri Sep 28, 2018 12:19 am
Re: HW Problem 4A.9
The most important concept to take into account here is that the energy it takes to heat up the water to its final temperature is equal to the amount of heat energy the copper loses. It can be portrayed as this:
ΔH(copper)=-ΔH(water)
After that, we refer to the general equation ΔH=m*C(specific)*ΔT and solve for the final temperature of water:
(20.0)(0.38)(T(final)-100.0)=-(50.7)(4.18)(T(f)-22.0)
Hope that helps!
ΔH(copper)=-ΔH(water)
After that, we refer to the general equation ΔH=m*C(specific)*ΔT and solve for the final temperature of water:
(20.0)(0.38)(T(final)-100.0)=-(50.7)(4.18)(T(f)-22.0)
Hope that helps!
-
Gisela F Ramirez 2H
- Posts: 61
- Joined: Fri Sep 28, 2018 12:27 am
Return to “Heat Capacities, Calorimeters & Calorimetry Calculations”
Who is online
Users browsing this forum: No registered users and 3 guests

