Hi,
During lecture the professor showed the process to derive the second order integrated rate law. However, I was confused about the integration part of the process, especially on the separate variables and integrate both sides part. Can someone walk me through the process in a bit more detailed way?
Thanks.
Integration
Moderators: Chem_Mod, Chem_Admin
-
OishiBhattacharya2K
- Posts: 100
- Joined: Fri Sep 24, 2021 6:03 am
Re: Integration
Take a first order reaction, where -d[A] /dt = k[A]
1st we must separate the variables, basically meaning move the "[A]" terms to one side, and the "dt" term to the other. This gives us d[A]/[A] = -k(dt)
Next, we integrate both sides using the rules of integration. One of the important rules of integration to know is the integral of 1/[x] dx = lnx+c. Using this info, we integrate both sides of our equation, giving us ln[A] = -kt+c. This makes sense because the integral of 1/[A] is ln[A], and the integral of a constant (in this case -k) is the constant times the variable (in this case -kt).
To find out what "c" is, set "t" equal to 0. This gives us the equation ln[A] = -kt+ln[A]initial
1st we must separate the variables, basically meaning move the "[A]" terms to one side, and the "dt" term to the other. This gives us d[A]/[A] = -k(dt)
Next, we integrate both sides using the rules of integration. One of the important rules of integration to know is the integral of 1/[x] dx = lnx+c. Using this info, we integrate both sides of our equation, giving us ln[A] = -kt+c. This makes sense because the integral of 1/[A] is ln[A], and the integral of a constant (in this case -k) is the constant times the variable (in this case -kt).
To find out what "c" is, set "t" equal to 0. This gives us the equation ln[A] = -kt+ln[A]initial
-
Nicole Ju 3H
- Posts: 110
- Joined: Fri Sep 24, 2021 6:20 am
Re: Integration
Another way you can look at the integration step is by setting the bounds to [A]initial and [A]t, and to t initial and t. This process is outlined in the textbook in section 7B. After integrating, you would get the same result as Ziyi's method.
-
Hanyi Jia 3B
- Posts: 118
- Joined: Fri Sep 24, 2021 6:08 am
- Been upvoted: 1 time
Re: Integration
The first step is writing out your differential rate law: r=k[A]2
This rate equals the unique rate for A, which is -d[A]/dt.
So you have -d[A]/dt=k[A]2
Rearrange: -d[A]/[A]2=kdt
Intergral both side: ∫-d[A]/[A]2=∫kdt, from (t0,[A0]) to (t,[A]).
Then it is just pure calculus.
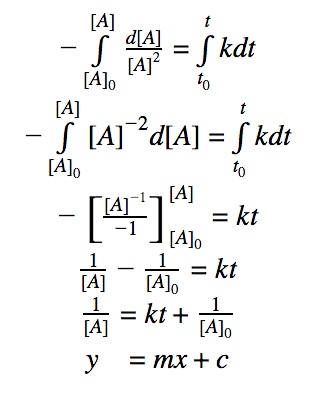
I assume you learned integration in the math class? Even if not, it is OK just to remember to integral rate law as you probably won't be asked to derive that.
Hope this helps.
This rate equals the unique rate for A, which is -d[A]/dt.
So you have -d[A]/dt=k[A]2
Rearrange: -d[A]/[A]2=kdt
Intergral both side: ∫-d[A]/[A]2=∫kdt, from (t0,[A0]) to (t,[A]).
Then it is just pure calculus.
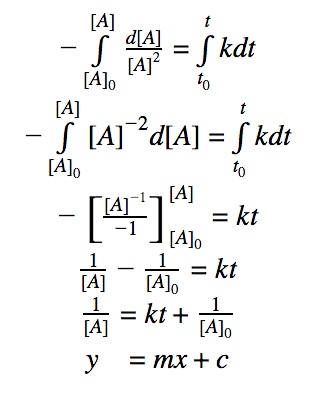
I assume you learned integration in the math class? Even if not, it is OK just to remember to integral rate law as you probably won't be asked to derive that.
Hope this helps.
Who is online
Users browsing this forum: No registered users and 0 guests

